Allergy Asthma Immunol Res.
2019 Jul;11(4):560-571. 10.4168/aair.2019.11.4.560.
Protease-Activated Receptors 2-Antagonist Suppresses Asthma by Inhibiting Reactive Oxygen Species-Thymic Stromal Lymphopoietin Inflammation and Epithelial Tight Junction Degradation
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University College of Veterinary Medicine, Gwangju, Korea.
- 2Asan Institute for Life Sciences, University of Ulsan College of Medicine, Seoul, Korea.
- 3CRID Center, NeoPharm Co., Ltd., Daejeon, Korea.
- 4Department of Pediatrics, Childhood Asthma Atopy Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sjhong@amc.seoul.kr
- 5Environmental Health Center, Asan Medical Center, Seoul, Korea.
- KMID: 2448758
- DOI: http://doi.org/10.4168/aair.2019.11.4.560
Abstract
- PURPOSE
Protease-activated receptor 2 (PAR2) reportedly triggers the immune response in allergic asthma. We aimed to investigate the mechanism on allergic inflammation mediated by PAR2.
METHODS
Human lung epithelial cells (A549 cells) were used for in vitro, and the German cockroach extract (GCE)-induced mouse model was developed for in vivo studies.
RESULTS
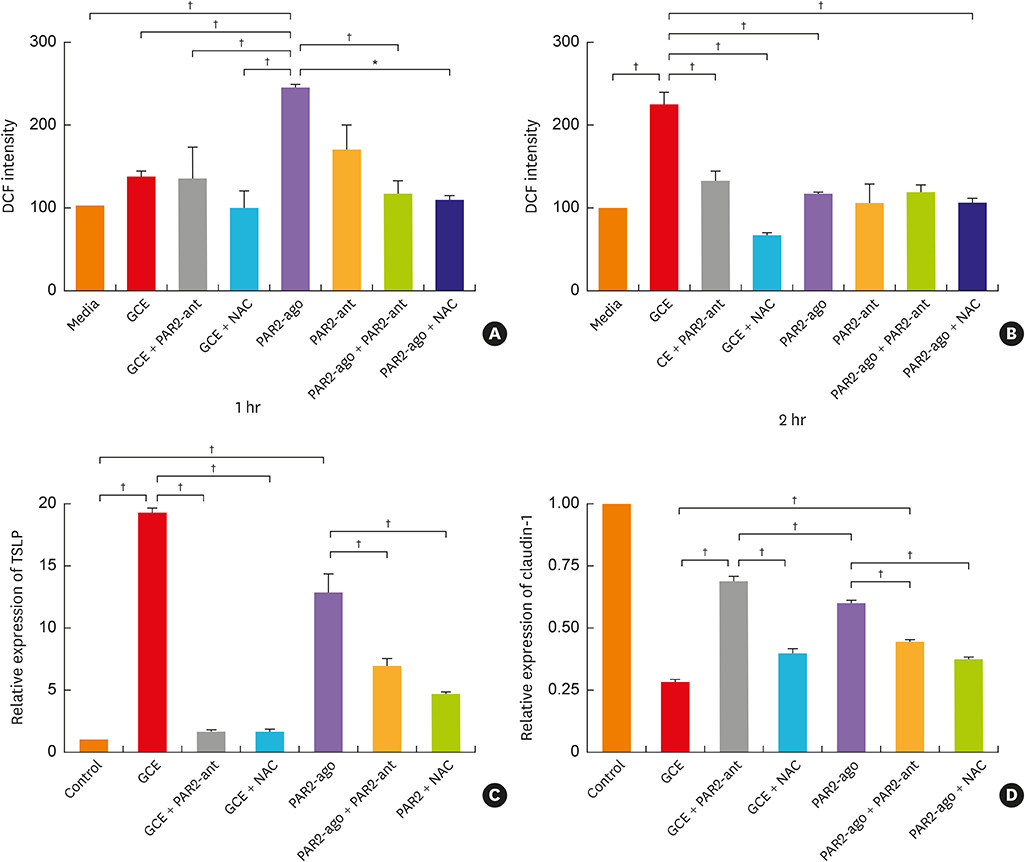
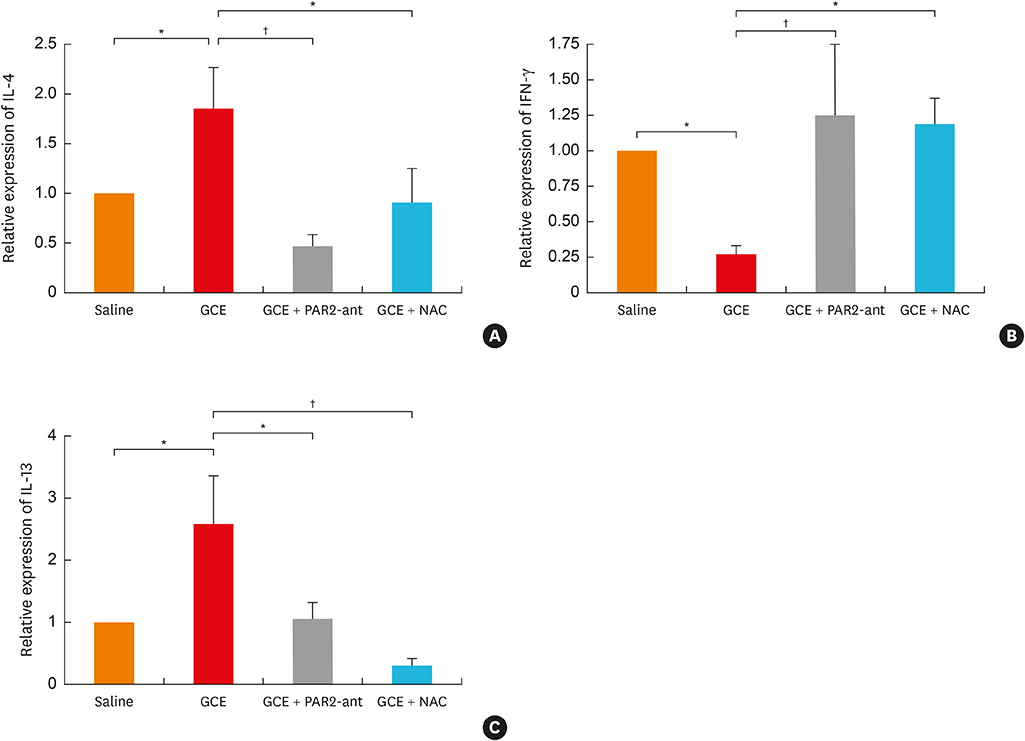
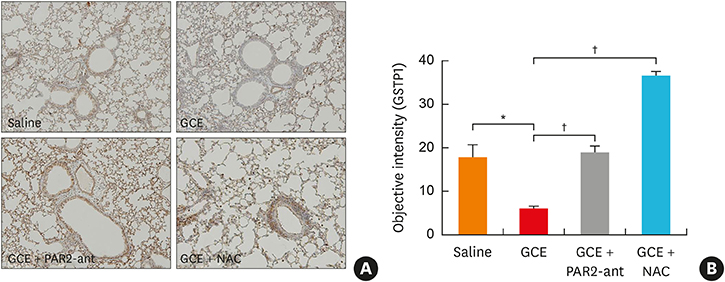
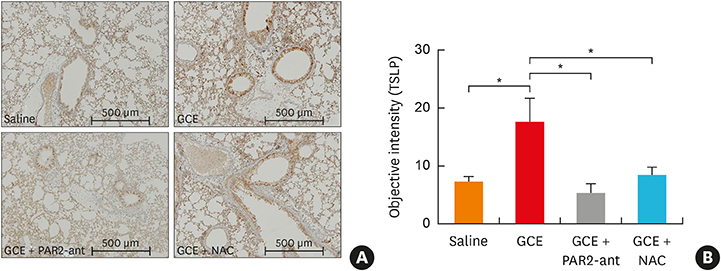
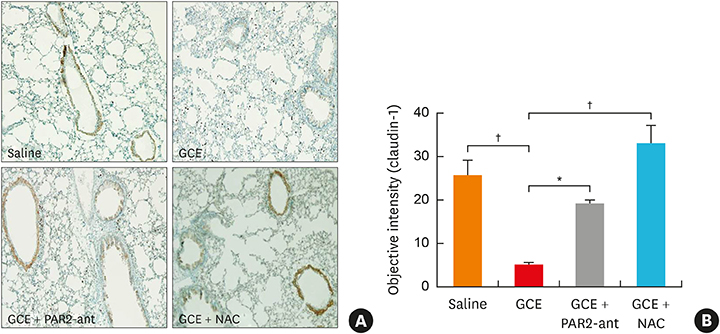
In A549 cells, the levels of reactive oxygen species (ROS) and thymic stromal lymphopoietin (TSLP) were significantly increased by GCE treatment, but were suppressed by PAR2-antagonist (PAR2-ant) or N-acetylcysteine (NAC) treatment. Claudin-1 was degraded by GCE, and was restored by PAR2-ant or NAC in the cells. In the mouse model, the clinical appearance including bronchial hyperresponsiveness, bronchoalveolar lavage fluid analysis and total immunoglobulin E were significantly suppressed by PAR2-ant or NAC. Moreover, TSLP levels in the lung were suppressed by the same treatments in the lung. Claudin-1 was also degraded by GCE, and was restored by PAR2-ant or NAC.
CONCLUSIONS
ROS generation and epidermal tight junction degradation are triggered by protease, followed by the induction of TSLP in allergic asthma. Our findings could suggest that PAR2-ant or anti-oxidants could be considered for allergic diseases as preventive alternatives.
Keyword
MeSH Terms
-
Acetylcysteine
Animals
Asthma*
Blattellidae
Bronchoalveolar Lavage Fluid
Claudin-1
Epithelial Cells
Humans
Immunoglobulin E
Immunoglobulins
In Vitro Techniques
Inflammation*
Lung
Mice
Oxygen*
Reactive Oxygen Species
Receptor, PAR-2
Receptors, Proteinase-Activated*
Tight Junctions*
Acetylcysteine
Claudin-1
Immunoglobulin E
Immunoglobulins
Oxygen
Reactive Oxygen Species
Receptor, PAR-2
Receptors, Proteinase-Activated
Figure
Cited by 1 articles
-
Critical Points on the Use of Biologicals in Allergic Diseases and Asthma
Ioana Agache, Catalina Cojanu, Alexandru Laculiceanu, Liliana Rogozea
Allergy Asthma Immunol Res. 2020;12(1):24-41. doi: 10.4168/aair.2020.12.1.24.
Reference
-
1. Milovanovic M, Heine G, Zuberbier T, Worm M. Allergen extract-induced interleukin-10 in human memory B cells inhibits immunoglobulin E production. Clin Exp Allergy. 2009; 39:671–678.
Article2. Traidl-Hoffmann C, Jakob T, Behrendt H. Determinants of allergenicity. J Allergy Clin Immunol. 2009; 123:558–566.
Article3. Chapman MD, Pomés A, Breiteneder H, Ferreira F. Nomenclature and structural biology of allergens. J Allergy Clin Immunol. 2007; 119:414–420.4. Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD. Targeting proteinase-activated receptors: therapeutic potential and challenges. Nat Rev Drug Discov. 2012; 11:69–86.
Article5. Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009; 183:1427–1434.
Article6. Kim HJ, Lee E, Lee SH, Kang MJ, Hong SJ. Mold elicits atopic dermatitis by reactive oxygen species: epidemiology and mechanism studies. Clin Immunol. 2015; 161:384–390.
Article7. Qu J, Li Y, Zhong W, Gao P, Hu C. Recent developments in the role of reactive oxygen species in allergic asthma. J Thorac Dis. 2017; 9:E32–E43.
Article8. Lin TJ, Karmaus WJJ, Chen ML, Hsu JC, Wang IJ. Interactions between bisphenol A exposure and GSTP1 polymorphisms in childhood asthma. Allergy Asthma Immunol Res. 2018; 10:172–179.9. Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010; 11:608–617.
Article10. Nadeem A, Alharbi NO, Vliagoftis H, Tyagi M, Ahmad SF, Sayed-Ahmed MM. Proteinase activated receptor-2-mediated dual oxidase-2 up-regulation is involved in enhanced airway reactivity and inflammation in a mouse model of allergic asthma. Immunology. 2015; 145:391–403.
Article11. Kauffman HF. Innate immune responses to environmental allergens. Clin Rev Allergy Immunol. 2006; 30:129–140.
Article12. Roche N, Stirling RG, Lim S, Oliver BG, Oates T, Jazrawi E, et al. Effect of acute and chronic inflammatory stimuli on expression of protease-activated receptors 1 and 2 in alveolar macrophages. J Allergy Clin Immunol. 2003; 111:367–373.
Article13. Shin YS, Sohn JH, Kim JY, Lee JH, Cho SH, Hong SJ, et al. Endotoxin is not essential for the development of cockroach induced allergic airway inflammation. Yonsei Med J. 2012; 53:593–602.
Article14. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006; 38:441–446.15. Moniaga CS, Jeong SK, Egawa G, Nakajima S, Hara-Chikuma M, Jeon JE, et al. Protease activity enhances production of thymic stromal lymphopoietin and basophil accumulation in flaky tail mice. Am J Pathol. 2013; 182:841–851.
Article16. Ying S, Meng Q, Corrigan CJ, Lee TH. Lack of filaggrin expression in the human bronchial mucosa. J Allergy Clin Immunol. 2006; 118:1386–1388.
Article17. Matsumura Y. Role of allergen source-derived proteases in sensitization via airway epithelial cells. J Allergy (Cairo). 2012; 2012:903659.
Article18. Jahnsen FL, Strickland DH, Thomas JA, Tobagus IT, Napoli S, Zosky GR, et al. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol. 2006; 177:5861–5867.
Article19. Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008; 8:193–204.
Article20. Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010; 10:225–235.
Article21. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010; 28:445–489.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Blockade of thymic stromal lymphopoietin and CRTH2 attenuates airway inflammation in a murine model of allergic asthma
- Protease Allergens Induce the Expression of IL-25 via Erk and p38 MAPK Pathway
- Allergy Inhibition Using Naturally Occurring Compounds Targeting Thymic Stromal Lymphopoietin Pathways: a Comprehensive Review
- Airway epithelial cells in airway inflammation and remodeling in asthma
- Role of Reactive Oxygen Species in Allergic Rhinitis