Int J Stem Cells.
2019 Mar;12(1):51-62. 10.15283/ijsc18061.
Generation of Organotypic Multicellular Spheres by Magnetic Levitation: Model for the Study of Human Hematopoietic Stem Cells Microenvironment
- Affiliations
-
- 1Immunobiology and Cell Biology Group, Department of Microbiology, Science Faculty, Pontificia Universidad Javeriana, Bogotá D.C., Colombia. vivianar@javeriana.edu.co
- 2Department of Pathology, School of Medicine, Pontificia Universidad Javeriana, Hospital Universitario San Ignacio, Bogotá D.C., Colombia.
- 3Virology Group, Department of Microbiology, Science Faculty, Pontificia Universidad Javeriana, Bogotá D.C., Colombia.
- 4Department of Gynecology and Obstetrics, School of Medicine, Pontificia Universidad Javeriana, Hospital Universitario San Ignacio, Bogotá D.C., Colombia.
- 5Department of Orthopedics and Traumatology, School of Medicine, Pontificia Universidad Javeriana, Hospital Universitario San Ignacio, Bogotá D.C., Colombia.
- KMID: 2447224
- DOI: http://doi.org/10.15283/ijsc18061
Abstract
- BACKGROUND AND OBJECTIVE
The characteristics of human hematopoietic stem cells are conditioned by the microenvironment of the bone marrow, where they interact with other cell populations, such as mesenchymal stem cells and endothelial cells; however, the study of this microenvironment is complex. The objective of this work was to develop a 3D culture system by magnetic levitation that imitates the microenvironment of human HSC.
METHODS AND RESULTS
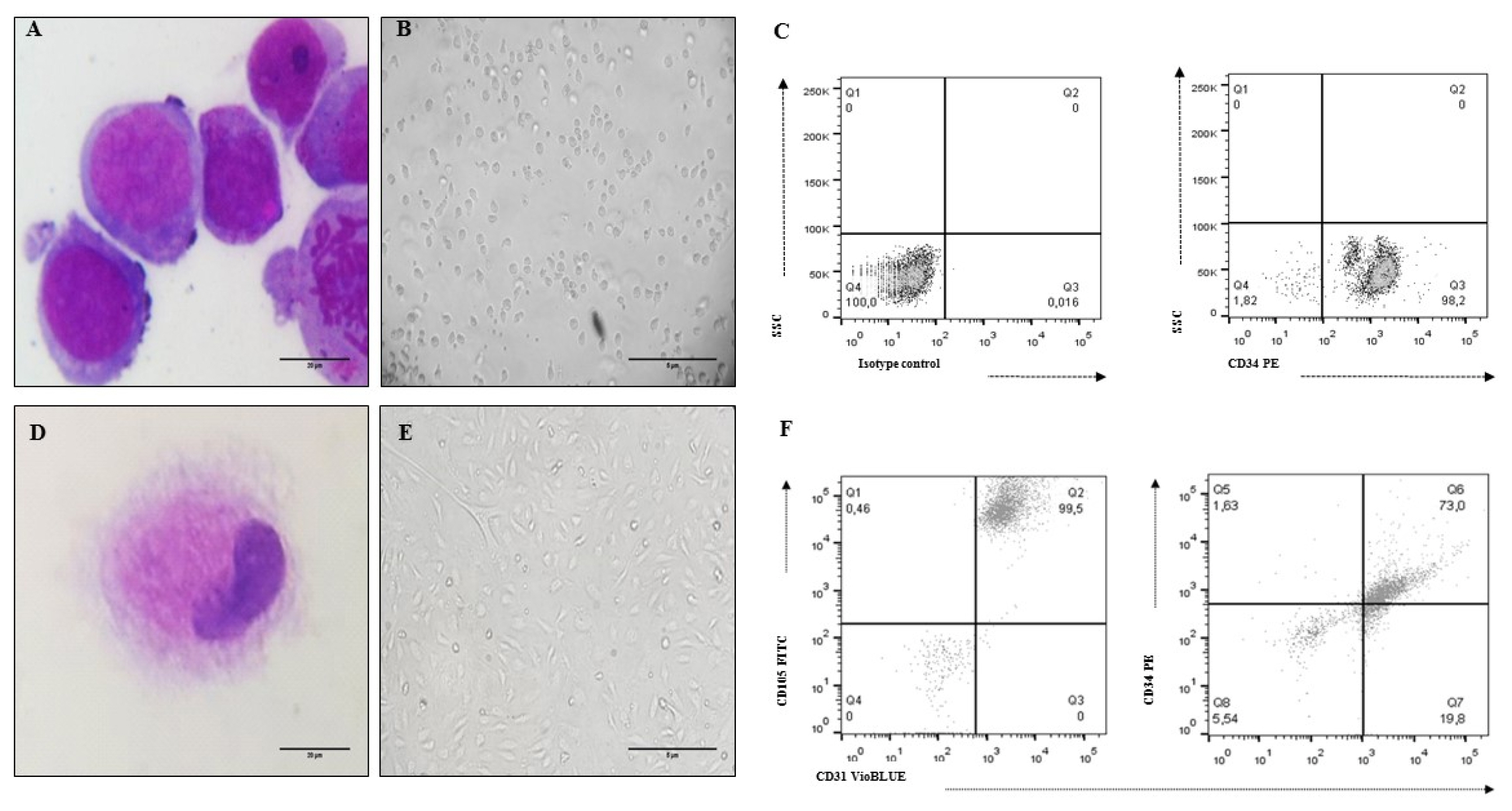
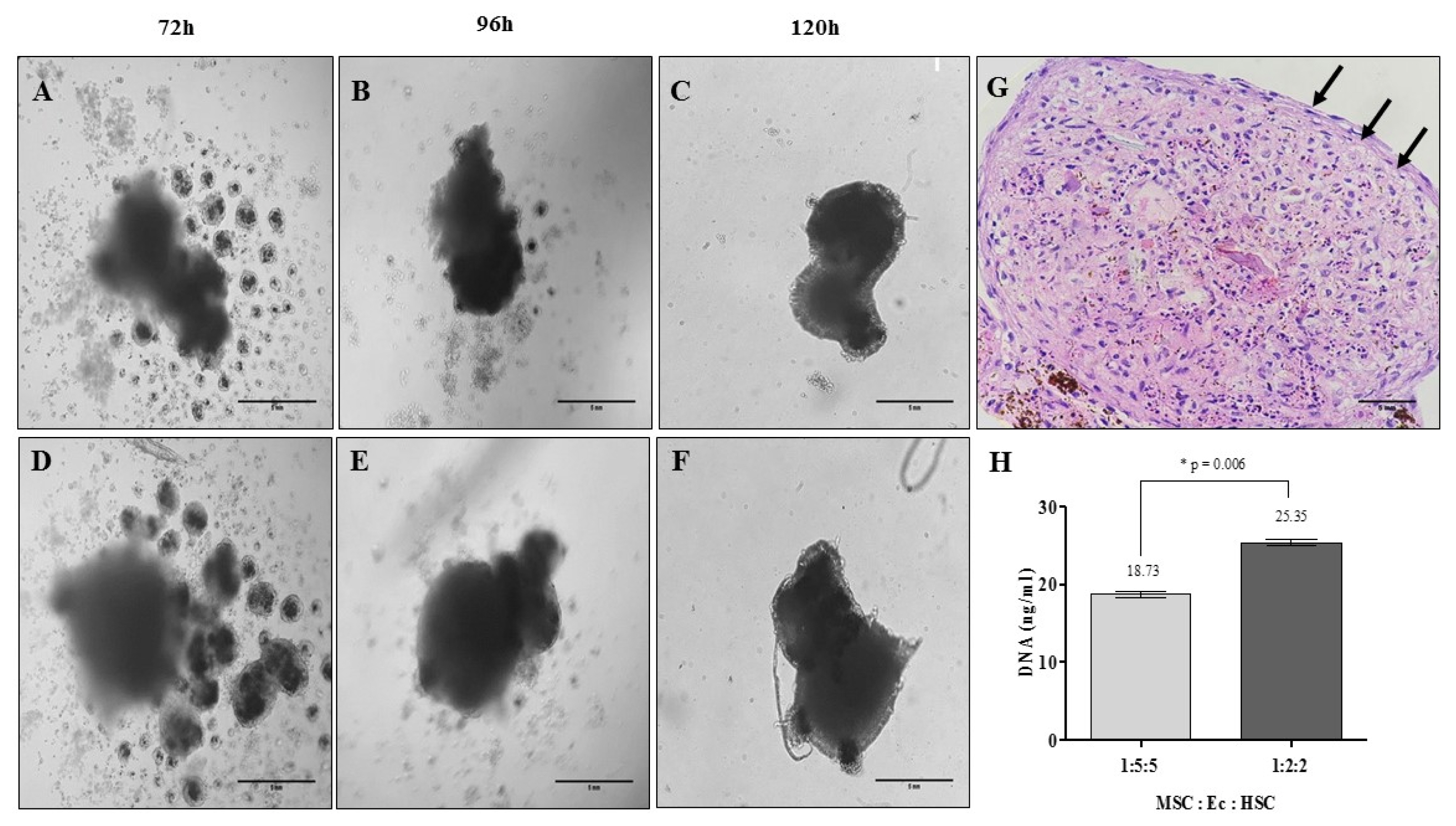
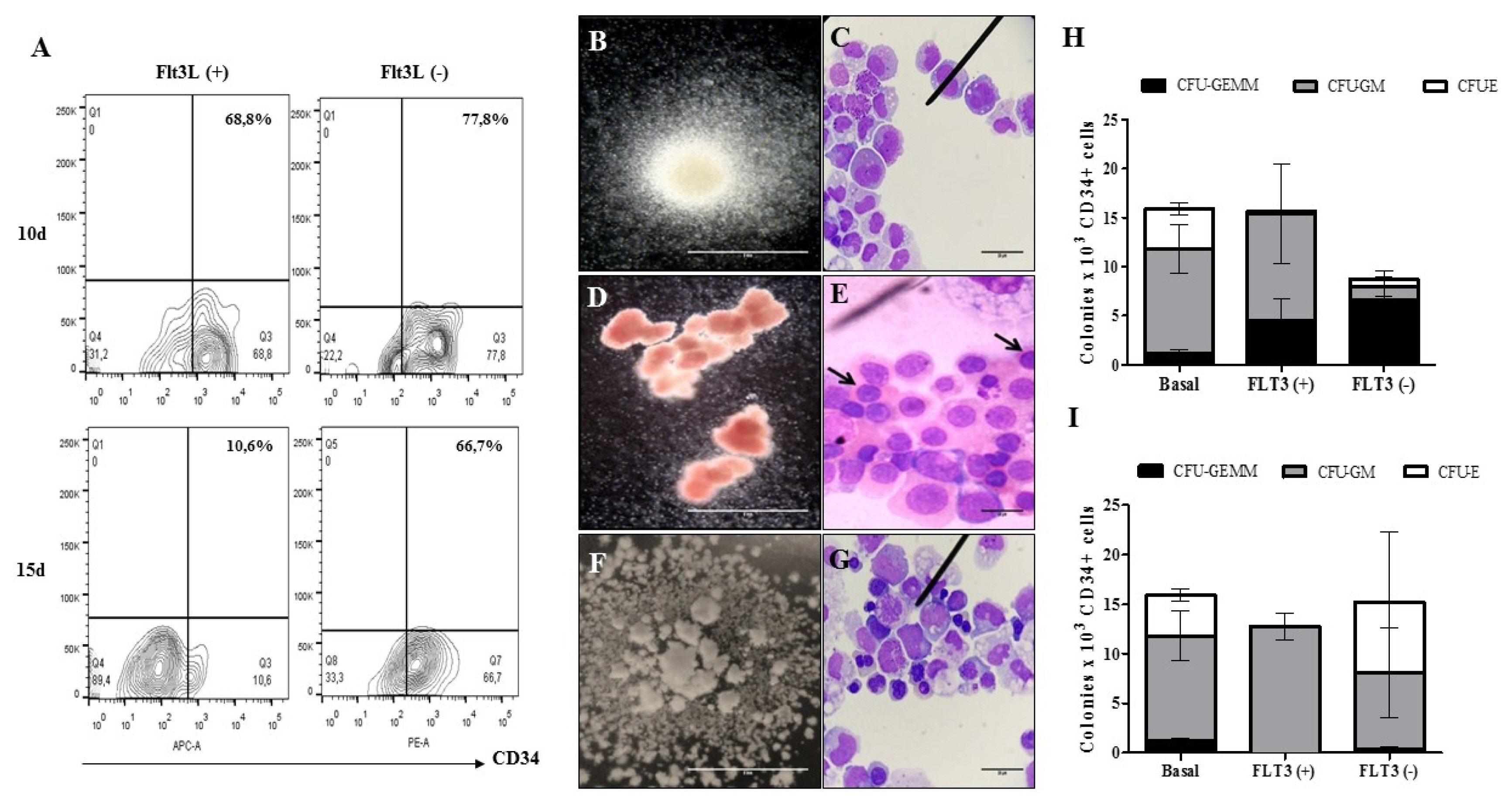
Human bone marrow-mesenchymal stem cells, umbilical cord blood-hematopoietic stem cells and a non-tumoral endothelial cell line (CC2811, Lonza®) were used to develop organotypic multicellular spheres by the magnetic levitation method. We obtained viable structures with an average sphericity index greater than 0.6, an average volume of 0.5 mm3 and a percentage of aggregation greater than 70%. Histological studies of the organotypic multicellular spheres used hematoxylin and eosin stains, and an evaluation of vimentin expression by means of immunohistochemistry demonstrated an organized internal structure without picnotic cells and a high expression of vimentin. The functional capacity of human hematopoietic stem cells after organotypic multicellular spheres culture was evaluated by multipotency tests, and it was demonstrated that 3D structures without exogenous Flt3L are autonomous in the maintenance of multipotency of human hematopoietic stem cells.
CONCLUSIONS
We developed organotypic multicellular spheres from normal human cells that mimic the microenvironment of the human hematopoietic stem cells. These structures are the prototype for the development of complex organoids that allow the further study of the biology of normal human stem cells and their potential in regenerative medicine.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978; 4:7–25. PMID: 747780.2. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014; 505:327–334. DOI: 10.1038/nature12984. PMID: 24429631. PMCID: 4514480.
Article3. Calvi LM, Link DC. The hematopoietic stem cell niche in homeostasis and disease. Blood. 2015; 126:2443–2451. DOI: 10.1182/blood-2015-07-533588. PMID: 26468230. PMCID: 4661168.
Article4. Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol. 2017; 19:214–223. DOI: 10.1038/ncb3475. PMID: 28218906. PMCID: 5467892.
Article5. Mangialardi G, Cordaro A, Madeddu P. The bone marrow pericyte: an orchestrator of vascular niche. Regen Med. 2016; 11:883–895. DOI: 10.2217/rme-2016-0121. PMID: 27885901. PMCID: 5677781.
Article6. Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013; 495:231–235. DOI: 10.1038/nature11885. PMID: 23434755. PMCID: 3600153.
Article7. Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, Rafii S. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010; 12:1046–1056. DOI: 10.1038/ncb2108. PMID: 20972423. PMCID: 2972406.
Article8. Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Lévesque JP. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012; 18:1651–1657. DOI: 10.1038/nm.2969. PMID: 23086476.
Article9. Chute JP, Muramoto GG, Dressman HK, Wolfe G, Chao NJ, Lin S. Molecular profile and partial functional analysis of novel endothelial cell-derived growth factors that regulate hematopoiesis. Stem Cells. 2006; 24:1315–1327. DOI: 10.1634/stemcells.2005-0029. PMID: 16373696.
Article10. Li W, Johnson SA, Shelley WC, Yoder MC. Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Exp Hematol. 2004; 32:1226–1237. DOI: 10.1016/j.exphem.2004.09.001. PMID: 15588947.
Article11. Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012; 481:457–462. DOI: 10.1038/nature10783. PMID: 22281595. PMCID: 3270376.
Article12. Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013; 495:227–230. DOI: 10.1038/nature11926. PMID: 23434756. PMCID: 3600148.
Article13. Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013; 502:637–643. DOI: 10.1038/nature12612. PMID: 24107994. PMCID: 3821873.
Article14. Rodríguez-Pardo VM, Aristizabal JA, Jaimes D, Quijano SM, de los Reyes I, Herrera MV, Solano J, Vernot JP. Mesenchymal stem cells promote leukaemic cells aberrant phenotype from B-cell acute lymphoblastic leukaemia. Hematol Oncol Stem Cell Ther. 2013; 6:89–100. DOI: 10.1016/j.hemonc.2013.09.002. PMID: 24161606.
Article15. Rodríguez-Pardo VM, Vernot JP. Mesenchymal stem cells promote a primitive phenotype CD34+c-kit+ in human cord blood-derived hematopoietic stem cells during ex vivo expansion. Cell Mol Biol Lett. 2013; 18:11–33. DOI: 10.2478/s11658-012-0036-1. PMID: 23104253. PMCID: 6275752.
Article16. Boieri M, Shah P, Dressel R, Inngjerdingen M. The role of animal models in the study of hematopoietic stem cell transplantation and GvHD: a historical overview. Front Immunol. 2016; 7:333. DOI: 10.3389/fimmu.2016.00333. PMID: 27625651. PMCID: 5003882.
Article17. Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010; 466:829–834. DOI: 10.1038/nature09262. PMID: 20703299. PMCID: 3146551.
Article18. Pinho S, Marchand T, Yang E, Wei Q, Nerlov C, Frenette PS. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev Cell. 2018; 44:634–641.e4. DOI: 10.1016/j.devcel.2018.01.016. PMID: 29456137. PMCID: 5886750.
Article19. Reichert D, Friedrichs J, Ritter S, Käubler T, Werner C, Bornhäuser M, Corbeil D. Phenotypic, morphological and adhesive differences of human hematopoietic progenitor cells cultured on murine versus human mesenchymal stromal cells. Sci Rep. 2015; 5:15680. DOI: 10.1038/srep15680. PMID: 26498381. PMCID: 4620509.
Article20. van Pel M, Fibbe WE, Schepers K. The human and murine hematopoietic stem cell niches: are they comparable? Ann N Y Acad Sci. 2016; 1370:55–64. DOI: 10.1111/nyas.12994. PMID: 26713726.
Article21. Simian M, Bissell MJ. Organoids: a historical perspective of thinking in three dimensions. J Cell Biol. 2017; 216:31–40. DOI: 10.1083/jcb.201610056. PMID: 28031422. PMCID: 5223613.
Article22. Jaganathan H, Gage J, Leonard F, Srinivasan S, Souza GR, Dave B, Godin B. Three-dimensional in vitro co-culture model of breast tumor using magnetic levitation. Sci Rep. 2014; 4:6468. DOI: 10.1038/srep06468. PMID: 25270048. PMCID: 4180823.
Article23. Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003; 83:173–180. DOI: 10.1002/bit.10655. PMID: 12768623.
Article24. Martin I, Dozin B, Quarto R, Cancedda R, Beltrame F. Computer-based technique for cell aggregation analysis and cell aggregation in in vitro chondrogenesis. Cytometry. 1997; 28:141–146. DOI: 10.1002/(SICI)1097-0320(19970601)28:2<141::AID-CYTO7>3.0.CO;2-I. PMID: 9181304.
Article25. Hordyjewska A, Popiołek Ł, Horecka A. Characteristics of hematopoietic stem cells of umbilical cord blood. Cytotechnology. 2015; 67:387–396. DOI: 10.1007/s10616-014-9796-y. PMID: 25373337. PMCID: 4371573.
Article26. Bari S, Seah KK, Poon Z, Cheung AM, Fan X, Ong SY, Li S, Koh LP, Hwang WY. Expansion and homing of umbilical cord blood hematopoietic stem and progenitor cells for clinical transplantation. Biol Blood Marrow Transplant. 2015; 21:1008–1019. DOI: 10.1016/j.bbmt.2014.12.022. PMID: 25555449.
Article27. Oubari F, Amirizade N, Mohammadpour H, Nakhlestani M, Zarif MN. The important role of FLT3-L in ex vivo expansion of hematopoietic stem cells following co-culture with mesenchymal stem cells. Cell J. 2015; 17:201–210. PMID: 26199899. PMCID: 4503834.28. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. DOI: 10.1080/14653240600855905. PMID: 16923606.
Article29. Moscona A, Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat. 1952; 86:287–301. PMID: 12980879. PMCID: 1273752.30. Abu-Absi SF, Friend JR, Hansen LK, Hu WS. Structural polarity and functional bile canaliculi in rat hepatocyte spheroids. Exp Cell Res. 2002; 274:56–67. DOI: 10.1006/excr.2001.5467. PMID: 11855857.
Article31. Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009; 30:2164–2174. DOI: 10.1016/j.biomaterials.2008.12.084. PMID: 19176247. PMCID: 3773699.
Article32. Robinson EE, Foty RA, Corbett SA. Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion. Mol Biol Cell. 2004; 15:973–981. DOI: 10.1091/mbc.e03-07-0528. PMID: 14718567. PMCID: 363054.33. Shimazui T, Schalken JA, Kawai K, Kawamoto R, van Bockhoven A, Oosterwijk E, Akaza H. Role of complex cadherins in cell-cell adhesion evaluated by spheroid formation in renal cell carcinoma cell lines. Oncol Rep. 2004; 11:357–360. PMID: 14719067.
Article34. Lee BH, Kim MH, Lee JH, Seliktar D, Cho NJ, Tan LP. Modulation of Huh7.5 spheroid formation and functionality using modified PEG-based hydrogels of different stiffness. PLoS One. 2015; 10:e0118123. DOI: 10.1371/journal.pone.0118123. PMID: 25692976. PMCID: 4333219.
Article35. Lin RZ, Chou LF, Chien CC, Chang HY. Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and beta1-integrin. Cell Tissue Res. 2006; 324:411–422. DOI: 10.1007/s00441-005-0148-2. PMID: 16489443.
Article36. Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia. 2015; 17:1–15. DOI: 10.1016/j.neo.2014.12.004. PMID: 25622895. PMCID: 4309685.
Article37. Morata-Tarifa C, Jiménez G, García MA, Entrena JM, Griñán-Lisón C, Aguilera M, Picon-Ruiz M, Marchal JA. Low adherent cancer cell subpopulations are enriched in tumorigenic and metastatic epithelial-to-mesenchymal transition-induced cancer stem-like cells. Sci Rep. 2016; 6:18772. DOI: 10.1038/srep18772. PMID: 26752044. PMCID: 4707518.
Article38. Haisler WL, Timm DM, Gage JA, Tseng H, Killian TC, Souza GR. Three-dimensional cell culturing by magnetic levitation. Nat Protoc. 2013; 8:1940–1949. DOI: 10.1038/nprot.2013.125. PMID: 24030442.
Article39. Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS, Bronk LF, Ananta JS, Mandelin J, Georgescu MM, Bankson JA, Gelovani JG, Killian TC, Arap W, Pasqualini R. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010; 5:291–296. DOI: 10.1038/nnano.2010.23. PMID: 20228788. PMCID: 4487889.
Article40. Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008; 3:1172–1184. DOI: 10.1002/biot.200700228. PMID: 18566957.
Article41. Goldman RD, Khuon S, Chou YH, Opal P, Steinert PM. The function of intermediate filaments in cell shape and cytoskeletal integrity. J Cell Biol. 1996; 134:971–983. DOI: 10.1083/jcb.134.4.971. PMID: 8769421. PMCID: 2120965.
Article42. Morishima N. Changes in nuclear morphology during apoptosis correlate with vimentin cleavage by different caspases located either upstream or downstream of Bcl-2 action. Genes Cells. 1999; 4:401–414. DOI: 10.1046/j.1365-2443.1999.00270.x. PMID: 10469173.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Generation of hematopoietic stem cells from human embryonic stem cells using a defined, stepwise, serum-free, and serum replacement-free monolayer culture method
- Glutathione Dynamics in the Tumor Microenvironment: A Potential Target of Cancer Stem Cells and T Cells
- The Fetal Sheep: A Unique Model System for Assessing the Full Differentiative Potential of Human Stem Cells
- Hormonal Regulation of Hematopoietic Stem Cells and Their Niche: A Focus on Estrogen