Blood Res.
2017 Mar;52(1):37-43. 10.5045/br.2017.52.1.37.
Generation of hematopoietic stem cells from human embryonic stem cells using a defined, stepwise, serum-free, and serum replacement-free monolayer culture method
- Affiliations
-
- 1Division of Intractable Diseases, Center for Biomedical Sciences, National Institute of Health and Korea Centers for Diseases Control and Prevention, Cheongju, Korea. kjhcorea@korea.kr
- 2Department of Biochemistry, College of Medicine, Chungbuk National University, Cheongju, Korea.
- KMID: 2375202
- DOI: http://doi.org/10.5045/br.2017.52.1.37
Abstract
- BACKGROUND
Embryonic stem cells (ESCs) can be expanded infinitely in vitro and have the potential to differentiate into hematopoietic stem cells (HSCs); thus, they are considered a useful source of cells for HSC production. Although several technical in vitro methods for engineering HSCs from pluripotent stem cells have been developed, clinical application of HSCs engineered from pluripotent stem cells is restricted because of the possibility of xenogeneic contamination resulting from the use of murine materials.
METHODS
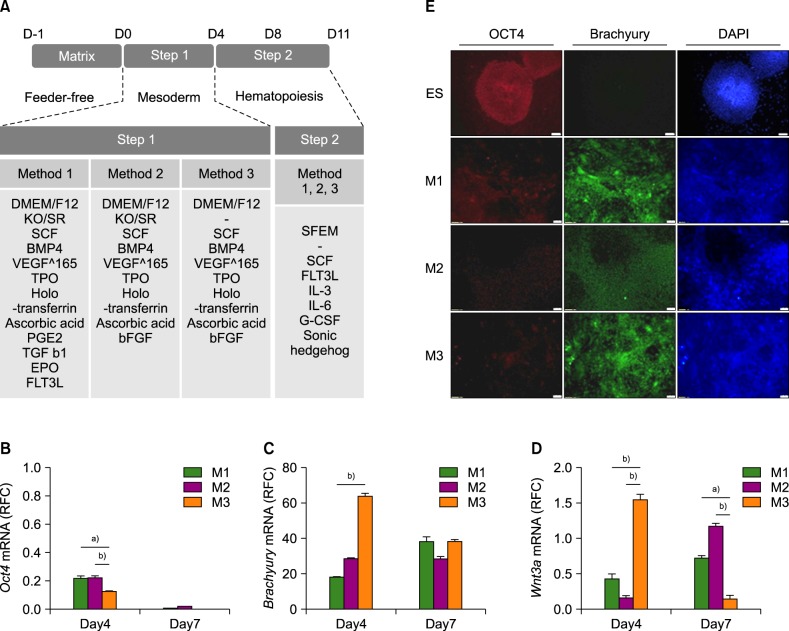
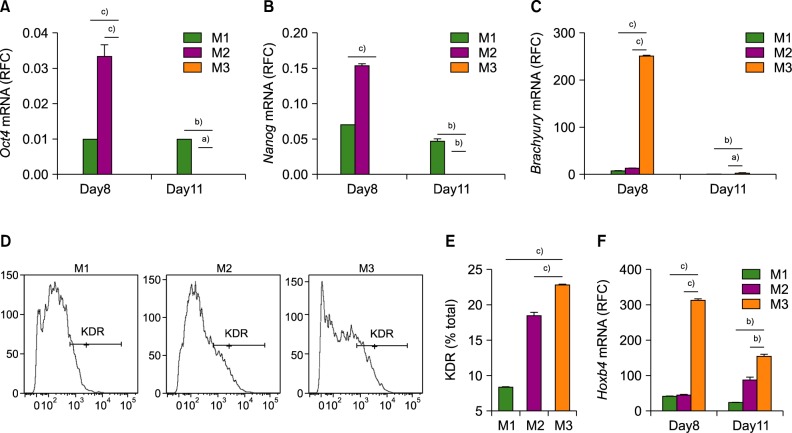
Human ESCs (CHA-hES15) were cultured on growth factor-reduced Matrigel-coated dishes in the mTeSR1 serum-free medium. When the cells were 70% confluent, we initiated HSC differentiation by three methods involving (1) knockout serum replacement (KSR), cytokines, TGFb1, EPO, and FLT3L; (2) KSR, cytokines, and bFGF; or (3) cytokines and bFGF.
RESULTS
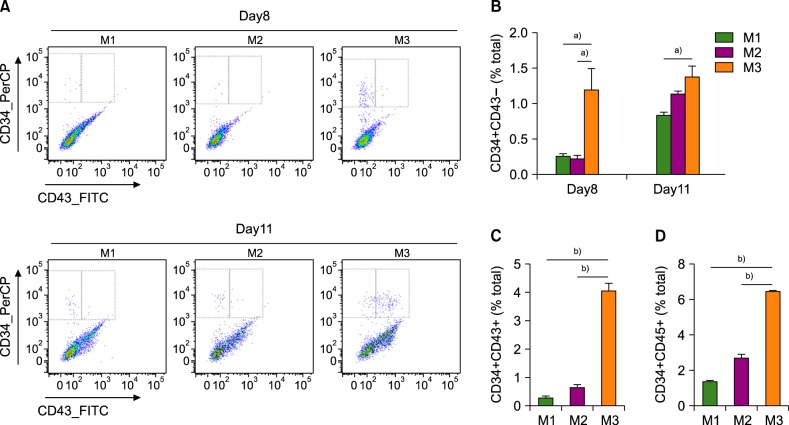
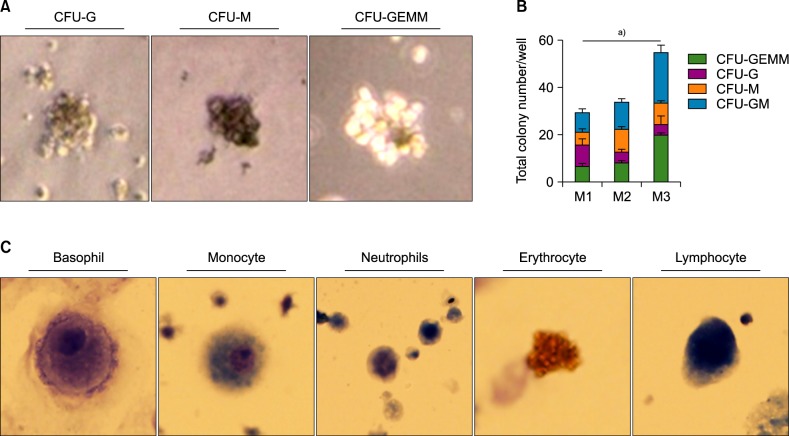
Among the three differentiation methods, the minimal number of cytokines without KSR resulted in the greatest production of HSCs. The optimized method resulted in a higher proportion of CD34âºCD43⺠hematopoietic progenitor cells (HPCs) and CD34âºCD45⺠HPCs compared to the other methods. In addition, the HSCs showed the potential to differentiate into multiple lineages of hematopoietic cells in vitro.
CONCLUSION
In this study, we optimized a two-step, serum-free, animal protein-free, KSR-free, feeder-free, chemically defined monolayer culture method for generation of HSCs and hematopoietic stem and progenitor cells (HSPCs) from human ESCs.
MeSH Terms
Figure
Reference
-
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147. PMID: 9804556.
Article2. Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001; 19:193–204. PMID: 11359944.
Article3. Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008; 132:661–680. PMID: 18295582.4. Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nat Rev Mol Cell Biol. 2016; 17:170–182. PMID: 26818440.
Article5. Grskovic M, Javaherian A, Strulovici B, Daley GQ. Induced pluripotent stem cells--opportunities for disease modelling and drug discovery. Nat Rev Drug Discov. 2011; 10:915–929. PMID: 22076509.6. Slukvin II. Hematopoietic specification from human pluripotent stem cells: current advances and challenges toward de novo generation of hematopoietic stem cells. Blood. 2013; 122:4035–4046. PMID: 24124087.
Article7. Suzuki N, Yamazaki S, Yamaguchi T, et al. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation. Mol Ther. 2013; 21:1424–1431. PMID: 23670574.
Article8. Amabile G, Welner RS, Nombela-Arrieta C, et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013; 121:1255–1264. PMID: 23212524.
Article9. Choi KD, Vodyanik M, Slukvin II. Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nat Protoc. 2011; 6:296–313. PMID: 21372811.
Article10. Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008; 3:85–98. PMID: 18593561.
Article11. Pang S, Wu Q, Tian S, et al. Establishment of a highly efficient hematopoietic differentiation model from human embryonic stem cells for functional screening. Sci China Life Sci. 2013; 56:1147–1149. PMID: 24302296.
Article12. Lee KY, Fong BS, Tsang KS, et al. Fetal stromal niches enhance human embryonic stem cell-derived hematopoietic differentiation and globin switch. Stem Cells Dev. 2011; 20:31–38. PMID: 20715903.
Article13. Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005; 105:617–626. PMID: 15374881.
Article14. Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006; 108:2095–2105. PMID: 16757688.
Article15. Aoyama K, Oritani K, Yokota T, et al. Stromal cell CD9 regulates differentiation of hematopoietic stem/progenitor cells. Blood. 1999; 93:2586–2594. PMID: 10194438.
Article16. Wang J, Zhao HP, Lin G, et al. In vitro hematopoietic differentiation of human embryonic stem cells induced by co-culture with human bone marrow stromal cells and low dose cytokines. Cell Biol Int. 2005; 29:654–661. PMID: 15950498.
Article17. Lim WF, Inoue-Yokoo T, Tan KS, Lai MI, Sugiyama D. Hematopoietic cell differentiation from embryonic and induced pluripotent stem cells. Stem Cell Res Ther. 2013; 4:71. PMID: 23796405.
Article18. Grigoriadis AE, Kennedy M, Bozec A, et al. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood. 2010; 115:2769–2776. PMID: 20065292.
Article19. Salvagiotto G, Burton S, Daigh CA, Rajesh D, Slukvin II, Seay NJ. A defined, feeder-free, serum-free system to generate in vitro hematopoietic progenitors and differentiated blood cells from hESCs and hiPSCs. PLoS One. 2011; 6:e17829. PMID: 21445267.
Article20. Niwa A, Heike T, Umeda K, et al. A novel serum-free monolayer culture for orderly hematopoietic differentiation of human pluripotent cells via mesodermal progenitors. PLoS One. 2011; 6:e22261. PMID: 21818303.
Article21. Lynch MR, Gasson JC, Paz H. Modified ES / OP9 co-culture protocol provides enhanced characterization of hematopoietic progeny. J Vis Exp. 2011; pii:2559.
Article22. Woods NB, Parker AS, Moraghebi R, et al. Brief report: efficient generation of hematopoietic precursors and progenitors from human pluripotent stem cell lines. Stem Cells. 2011; 29:1158–1164. PMID: 21544903.
Article23. Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001; 2:172–180. PMID: 11175816.
Article24. Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011; 117:6083–6090. PMID: 21436068.
Article25. Biancotti JC, Town T. Increasing hematopoietic stem cell yield to develop mice with human immune systems. Biomed Res Int. 2013; 2013:740892. PMID: 23509770.
Article26. Gertow K, Hirst CE, Yu QC, et al. WNT3A promotes hematopoietic or mesenchymal differentiation from hESCs depending on the time of exposure. Stem Cell Reports. 2013; 1:53–65. PMID: 24052942.
Article27. Ziegler BL, Valtieri M, Porada GA, et al. KDR receptor: a key marker defining hematopoietic stem cells. Science. 1999; 285:1553–1558. PMID: 10477517.
Article28. Lian X, Bao X, Al-Ahmad A, et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports. 2014; 3:804–816. PMID: 25418725.
Article29. Sarma NJ, Takeda A, Yaseen NR. Colony forming cell (CFC) assay for human hematopoietic cells. J Vis Exp. 2010; –pii:2195.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Serum-Free Media in RBC Differentiation from Human Hematopoietic Stem Cells
- Maintenance of hPSCs under Xeno-Free and Chemically Defined Culture Conditions
- Engineering Biomaterials for Feeder-Free Maintenance of Human Pluripotent Stem Cells
- Hematopoietic Stem Cells Culture, Expansion and Differentiation: An Insight into Variable and Available Media
- Current Concepts of Stem Cell Therapy