J Breast Cancer.
2018 Mar;21(1):11-20. 10.4048/jbc.2018.21.1.11.
Isomangiferin, a Novel Potent Vascular Endothelial Growth Factor Receptor 2 Kinase Inhibitor, Suppresses Breast Cancer Growth, Metastasis and Angiogenesis
- Affiliations
-
- 1Research Center of Basic Medical Sciences, School of Basic Medical Sciences, Hubei University of Science and Technology, Xianning, China. humeichun.530@163.com
- 2Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China.
- 3Cancer Center, Sanford Burnham Prebys Medical Discovery Institute, La Jolla, USA.
- 4Department of Optoelectronic Engineering, School of Electrical and Information Engineering, Hubei University of Science and Technology, Xianning, China.
- KMID: 2441863
- DOI: http://doi.org/10.4048/jbc.2018.21.1.11
Abstract
- PURPOSE
Vascular endothelial growth factor (VEGF) signal transduction mainly depends on its binding to VEGF receptor 2 (VEGFR-2). VEGF downstream signaling proteins mediate several of its effects in cancer progression, including those on tumor growth, metastasis, and blood vessel formation. The activation of VEGFR-2 signaling is a hallmark of and is considered a therapeutic target for breast cancer. Here, we report a study of the regulation of the VEGFR-2 signaling pathway by a small molecule, isomangiferin.
METHODS
A human breast cancer xenograft mouse model was used to investigate the efficacy of isomangiferin in vivo. The inhibitory effect of isomangiferin on breast cancer cells and the underlying mechanism were examined in vitro.
RESULTS
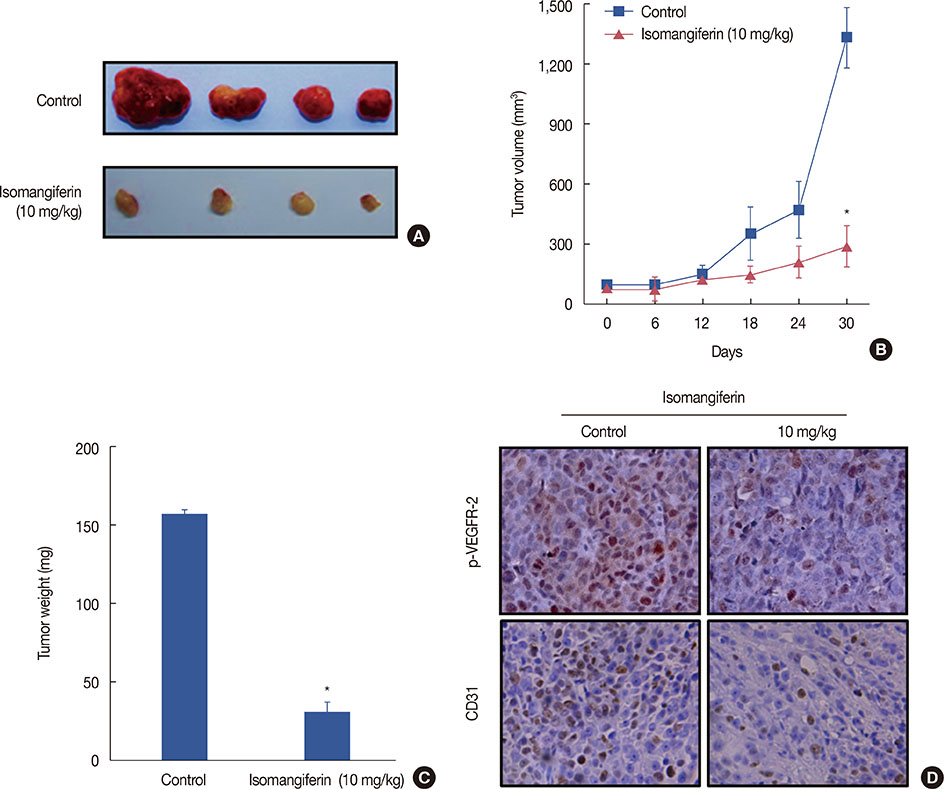
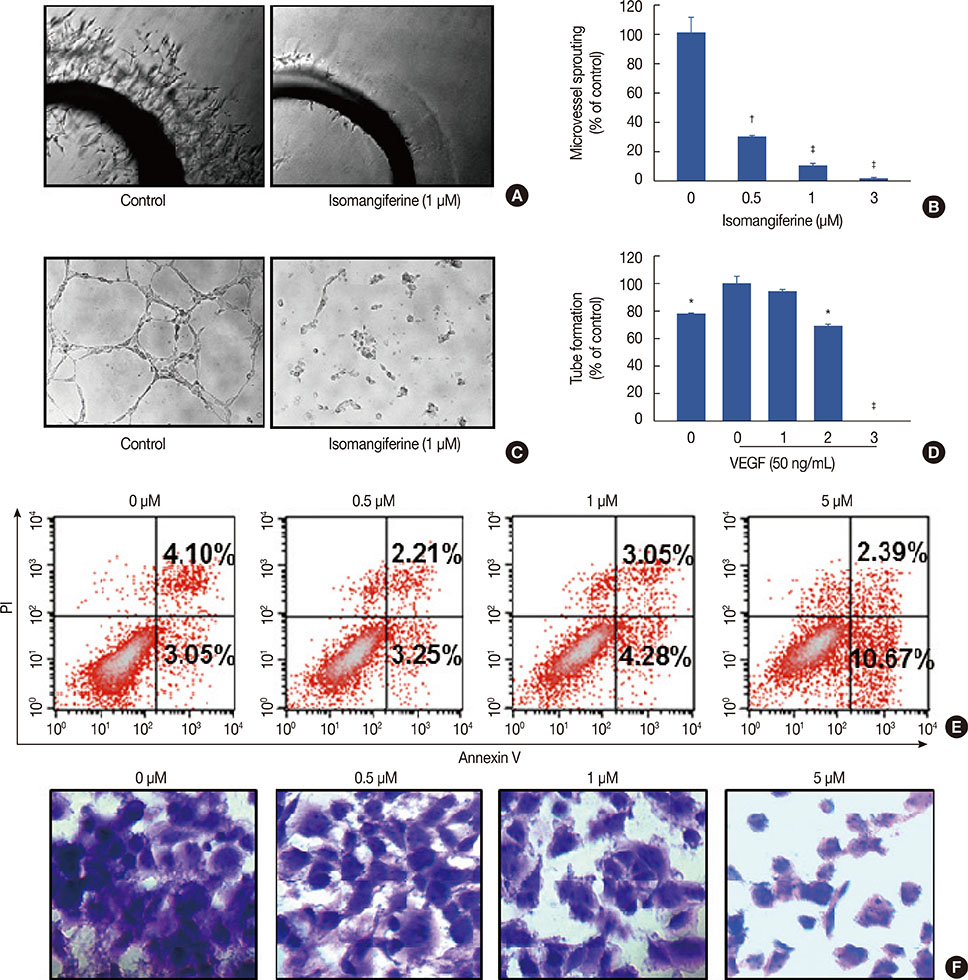
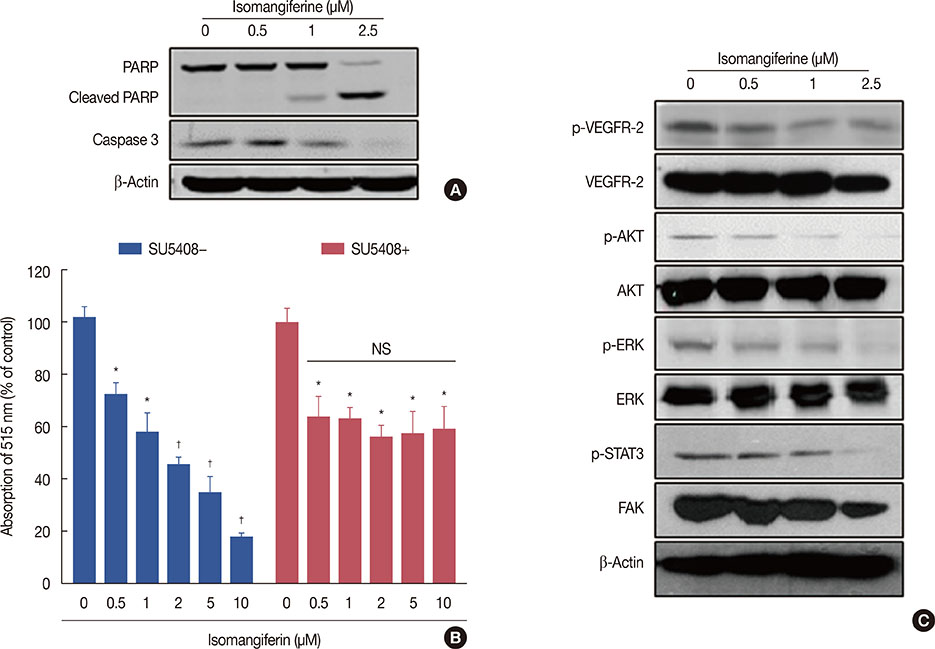
Isomangiferin suppressed tumor growth in xenografts. In vitro, isomangiferin treatment inhibited cancer cell proliferation, migration, invasion, and adhesion. The effect of isomangiferin on breast cancer growth was well coordinated with its suppression of angiogenesis. A rat aortic ring assay revealed that isomangiferin significantly inhibited blood vessel formation during VEGF-induced microvessel sprouting. Furthermore, isomangiferin treatment inhibited VEGF-induced proliferation of human umbilical vein endothelial cells and the formation of capillary-like structures. Mechanistically, isomangiferin induced caspase-dependent apoptosis of breast cancer cells. Furthermore, VEGF-induced activation of the VEGFR-2 kinase pathway was down-regulated by isomangiferin.
CONCLUSION
Our findings demonstrate that isomangiferin exerts anti-breast cancer effects via the functional inhibition of VEGFR-2. Pharmaceutically targeting VEGFR-2 by isomangiferin could be an effective therapeutic strategy for breast cancer.
Keyword
MeSH Terms
-
Angiogenesis Inhibitors
Animals
Apoptosis
Blood Vessels
Breast Neoplasms
Cell Proliferation
Heterografts
Human Umbilical Vein Endothelial Cells
Humans
In Vitro Techniques
Mice
Microvessels
Neoplasm Metastasis
Phosphotransferases
Rats
Receptors, Vascular Endothelial Growth Factor*
Signal Transduction
Vascular Endothelial Growth Factor A*
Vascular Endothelial Growth Factor Receptor-2*
Angiogenesis Inhibitors
Phosphotransferases
Receptors, Vascular Endothelial Growth Factor
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-2
Figure
Reference
-
1. Jandial R, Hoshide R, Waters JD, Somlo G. Operative and therapeutic advancements in breast cancer metastases to the brain. Clin Breast Cancer. Epub 2017 Oct 7. DOI: 10.1016/j.clbc.2017.10.002.
Article2. Moreno AC, Lin YH, Bedrosian I, Shen Y, Stauder MC, Smith BD, et al. Use of regional nodal irradiation and its association with survival for women with high-risk, early stage breast cancer: a national cancer database analysis. Adv Radiat Oncol. 2017; 2:291–300.
Article3. Santoni M, Guerra F, Conti A, Lucarelli A, Rinaldi S, Belvederesi L, et al. Incidence and risk of cardiotoxicity in cancer patients treated with targeted therapies. Cancer Treat Rev. 2017; 59:123–131.
Article4. Zhou J, Wang H, Zhang H, Lutz AM, Tian L, Hristov D, et al. VEGFR2-targeted three-dimensional ultrasound imaging can predict responses to antiangiogenic therapy in preclinical models of colon cancer. Cancer Res. 2016; 76:4081–4089.
Article5. Pan Y, Zheng M, Zhong L, Yang J, Zhou S, Qin Y, et al. A preclinical evaluation of SKLB261, a multikinase inhibitor of EGFR/Src/VEGFR2, as a therapeutic agent against pancreatic cancer. Mol Cancer Ther. 2015; 14:407–418.
Article6. Maione P, Sgambato A, Casaluce F, Sacco PC, Santabarbara G, Rossi A, et al. The role of the antiangiogenetic ramucirumab in the treatment of advanced non small cell lung cancer. Curr Med Chem. 2017; 24:3–13.
Article7. Longatto-Filho A, Pinheiro C, Martinho O, Moreira MA, Ribeiro LF, Queiroz GS, et al. Molecular characterization of EGFR, PDGFRA and VEGFR2 in cervical adenosquamous carcinoma. BMC Cancer. 2009; 9:212.
Article8. Dunna NR, Kandula V, Girdhar A, Pudutha A, Hussain T, Bandaru S, et al. High affinity pharmacological profiling of dual inhibitors targeting RET and VEGFR2 in inhibition of kinase and angiogeneis events in medullary thyroid carcinoma. Asian Pac J Cancer Prev. 2015; 16:7089–7095.
Article9. Szabo E, Schneider H, Seystahl K, Rushing EJ, Herting F, Weidner KM, et al. Autocrine VEGFR1 and VEGFR2 signaling promotes survival in human glioblastoma models in vitro and in vivo. Neuro Oncol. 2016; 18:1242–1252.
Article10. Sato H, Takeda Y. VEGFR2 expression and relationship between tumor neovascularization and histologic characteristics in oral squamous cell carcinoma. J Oral Sci. 2009; 51:551–557.
Article11. Zhang J, Liu C, Shi W, Yang L, Zhang Q, Cui J, et al. The novel VEGF receptor 2 inhibitor YLL545 inhibits angiogenesis and growth in breast cancer. Oncotarget. 2016; 7:41067–41080.
Article12. Zhu X, Zhou W. The emerging regulation of VEGFR-2 in triple-negative breast cancer. Front Endocrinol (Lausanne). 2015; 6:159.
Article13. Yan JD, Liu Y, Zhang ZY, Liu GY, Xu JH, Liu LY, et al. Expression and prognostic significance of VEGFR-2 in breast cancer. Pathol Res Pract. 2015; 211:539–543.
Article14. Islam MN, Yoo HH, Lee J, Nam JW, Seo EK, Jin C, et al. Simultaneous determination of bioactive xanthone glycosides and norlignans from ethanolic extract of Anemarrhena asphodeloides by liquid chromatography. J AOAC Int. 2008; 91:1271–1277.
Article15. Satish Rao BS, Sreedevi MV, Nageshwar Rao B. Cytoprotective and antigenotoxic potential of Mangiferin, a glucosylxanthone against cadmium chloride induced toxicity in HepG2 cells. Food Chem Toxicol. 2009; 47:592–600.
Article16. Shen J, Song G, An M, Li X, Wu N, Ruan K, et al. The use of hollow mesoporous silica nanospheres to encapsulate bortezomib and improve efficacy for non-small cell lung cancer therapy. Biomaterials. 2014; 35:316–326.
Article17. Song G, Shen J, Jiang F, Hu R, Li W, An L, et al. Hydrophilic molybdenum oxide nanomaterials with controlled morphology and strong plasmonic absorption for photothermal ablation of cancer cells. ACS Appl Mater Interfaces. 2014; 6:3915–3922.
Article18. Li C, Hu J, Li W, Song G, Shen J. Combined bortezomib-based chemotherapy and p53 gene therapy using hollow mesoporous silica nanospheres for p53 mutant non-small cell lung cancer treatment. Biomater Sci. 2016; 5:77–88.
Article19. Shen J, Ma H, Zhang T, Liu H, Yu L, Li G, et al. Magnolol inhibits the growth of non-small cell lung cancer via inhibiting microtubule polymerization. Cell Physiol Biochem. 2017; 42:1789–1801.
Article20. Shen J, Sheng X, Chang Z, Wu Q, Wang S, Xuan Z, et al. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep. 2014; 7:180–193.
Article21. Shen J, Zhang S, Li Y, Zhang W, Chen J, Zhang M, et al. p14(ARF) inhibits the functions of adenovirus E1A oncoprotein. Biochem J. 2011; 434:275–285.
Article22. Yamada KH, Kang H, Malik AB. Antiangiogenic therapeutic potential of peptides derived from the molecular motor KIF13B that transports VEGFR2 to plasmalemma in endothelial cells. Am J Pathol. 2017; 187:214–224.
Article23. Varga A, Gyulavári P, Greff Z, Futosi K, Németh T, Simon-Szabó L, et al. Targeting vascular endothelial growth factor receptor 2 and protein kinase D1 related pathways by a multiple kinase inhibitor in angiogenesis and inflammation related processes in vitro. PLoS One. 2015; 10:e0124234.
Article24. Chen H, Cong Q, Du Z, Liao W, Zhang L, Yao Y, et al. Sulfated fucoidan FP08S2 inhibits lung cancer cell growth in vivo by disrupting angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Cancer Lett. 2016; 382:44–52.
Article25. DeWire M, Fouladi M, Turner DC, Wetmore C, Hawkins C, Jacobs C, et al. An open-label, two-stage, phase II study of bevacizumab and lapatinib in children with recurrent or refractory ependymoma: a collaborative ependymoma research network study (CERN). J Neurooncol. 2015; 123:85–91.
Article26. Liu H, Wu B, Pan G, He L, Li Z, Fan M, et al. Metabolism and pharmacokinetics of mangiferin in conventional rats, pseudo-germ-free rats, and streptozotocin-induced diabetic rats. Drug Metab Dispos. 2012; 40:2109–2118.
Article27. Zhao J, Zhang B, Li S, Zeng L, Chen Y, Fang J. Mangiferin increases Nrf2 protein stability by inhibiting its ubiquitination and degradation in human HL60 myeloid leukemia cells. Int J Mol Med. 2014; 33:1348–1354.
Article28. Dilshara MG, Kang CH, Choi YH, Kim GY. Mangiferin inhibits tumor necrosis factor-alpha-induced matrix metalloproteinase-9 expression and cellular invasion by suppressing nuclear factor-kappaB activity. BMB Rep. 2015; 48:559–564.
Article29. Núñez Selles AJ, Daglia M, Rastrelli L. The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. Biofactors. 2016; 42:475–491.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Vascular Endothelial Growth Factor-C in Breast Carcinoma
- The Relationship between PTEN Tumor Suppressor Gene and Vascular Endothelial Growth Factor-Mediated Angiogenesis in Breast Cancer
- Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis
- Zerumbone, Sesquiterpene Photochemical from Ginger, Inhibits Angiogenesis
- Thrombospondin-1 and Inhibition of Tumor Growth