Korean J Physiol Pharmacol.
2015 Jul;19(4):335-340. 10.4196/kjpp.2015.19.4.335.
Zerumbone, Sesquiterpene Photochemical from Ginger, Inhibits Angiogenesis
- Affiliations
-
- 1Department of Biomedical Science, Catholic University of Daegu, Gyeongsan 712-702, Korea. toto0818@cu.ac.kr
- KMID: 2285576
- DOI: http://doi.org/10.4196/kjpp.2015.19.4.335
Abstract
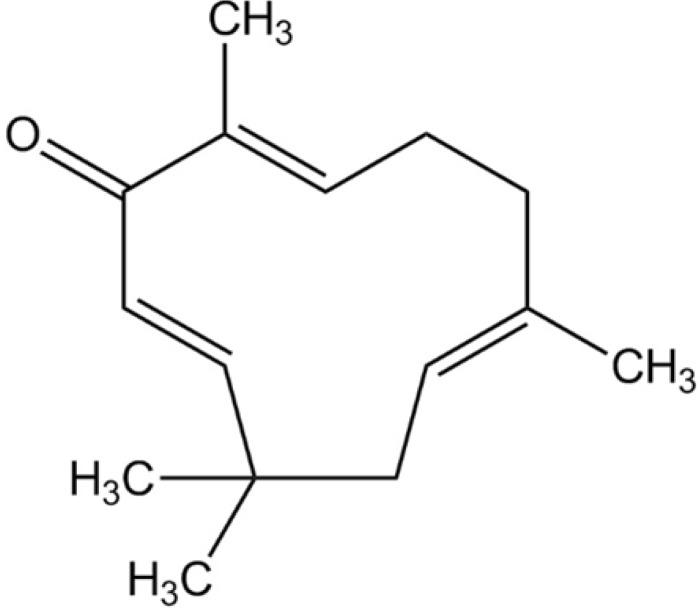
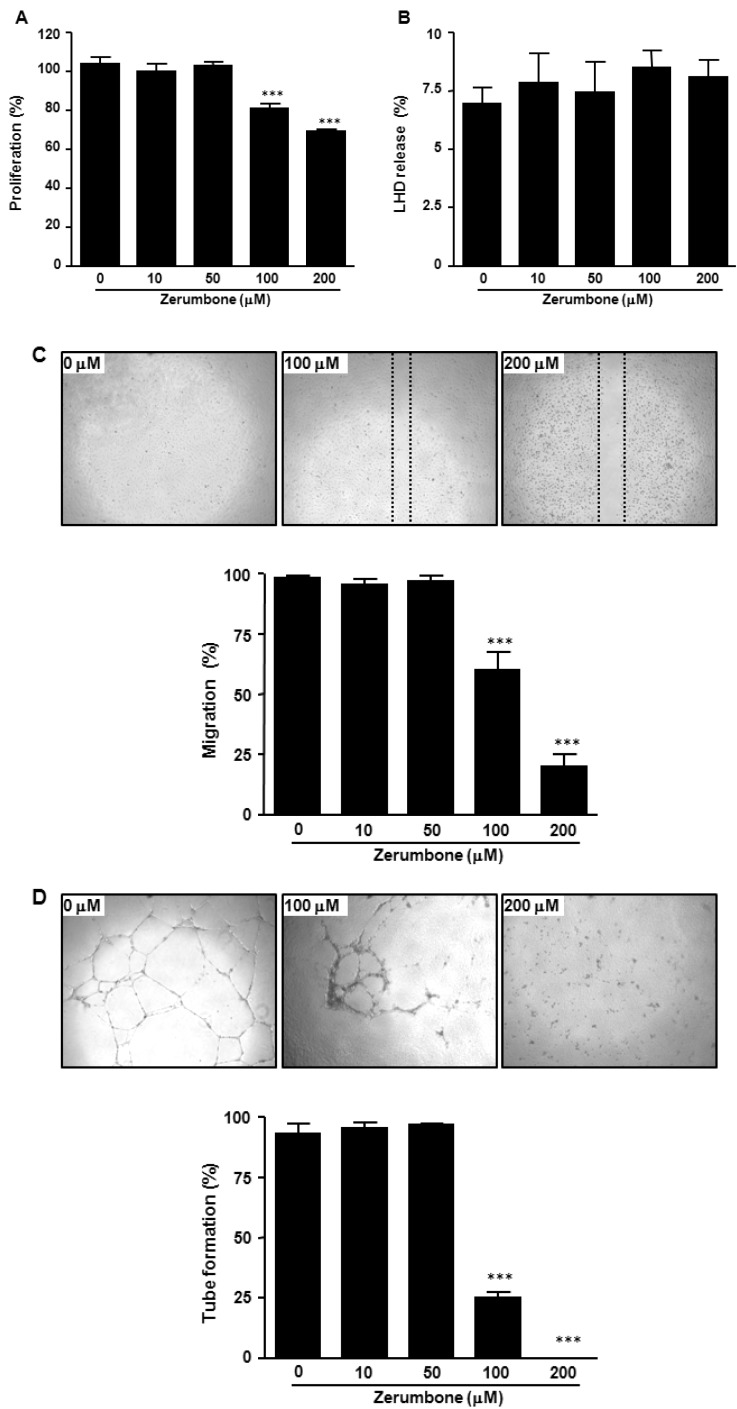
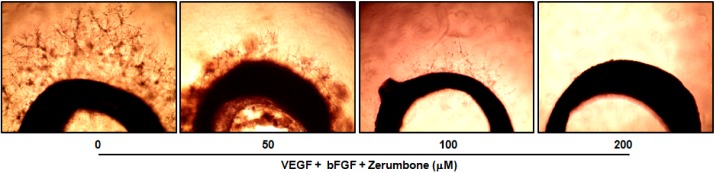
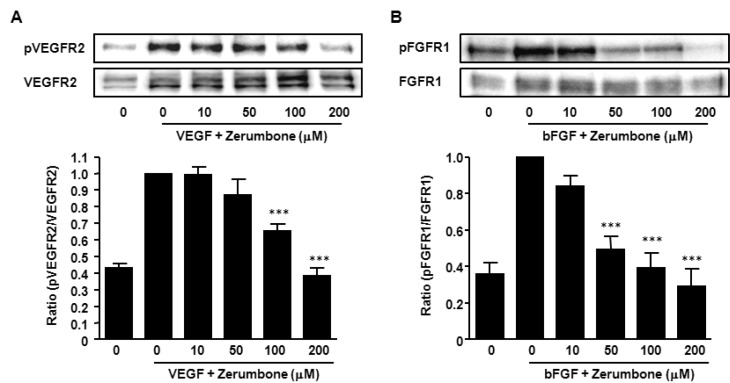
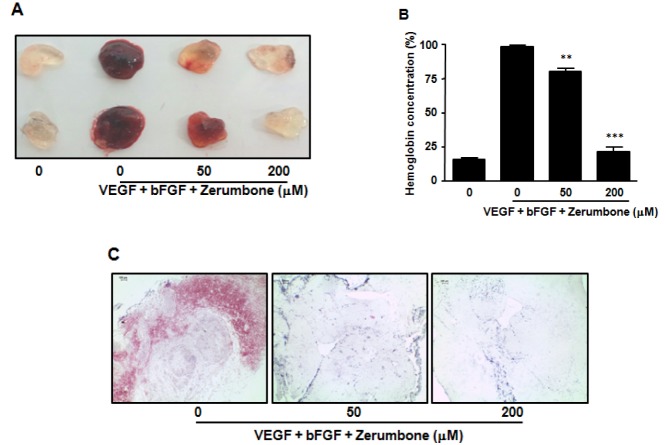
- Here, we investigated the role of zerumbone, a natural cyclic sesquiterpene of Zingiber zerumbet Smith, on angiogenesis using human umbilical vein endothelial cells (HUVECs). Zerumbone inhibited HUVECs proliferation, migration and tubule formation, as well as angiogenic activity by rat aorta explants. In particular, zerumbone inhibited phosphorylation of vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1, which are key regulators of endothelial cell function and angiogenesis. In vivo matrigel plug assay in mice demonstrated significant decrease in vascularization and hemoglobin content in the plugs from zerumbone-treated mice, compared with control mice. Overall, these results suggest that zerumbone inhibits various attributes of angiogenesis, which might contribute to its reported antitumor effects.
Keyword
MeSH Terms
-
Animals
Aorta
Endothelial Cells
Fibroblast Growth Factors
Ginger*
Human Umbilical Vein Endothelial Cells
Mice
Phosphorylation
Rats
Receptors, Vascular Endothelial Growth Factor
Vascular Endothelial Growth Factor Receptor-2
Fibroblast Growth Factors
Receptors, Vascular Endothelial Growth Factor
Vascular Endothelial Growth Factor Receptor-2
Figure
Reference
-
1. Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013; 4:384S–392S. PMID: 23674808.
Article2. Key TJ, Appleby PN, Crowe FL, Bradbury KE, Schmidt JA, Travis RC. Cancer in British vegetarians: updated analyses of 4998 incident cancers in a cohort of 32,491 meat eaters, 8612 fish eaters, 18,298 vegetarians, and 2246 vegans. Am J Clin Nutr. 2014; 100(Suppl 1):378S–385S. PMID: 24898235.
Article3. Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002; 76:1191–1201. PMID: 12450882.
Article4. Canter PH, Thomas H, Ernst E. Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 2005; 23:180–185. PMID: 15780709.
Article5. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000; 407:249–257. PMID: 11001068.
Article6. Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986; 83:7297–7301. PMID: 2429303.
Article7. Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013; 13:871–882. PMID: 24263190.
Article8. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003; 9:653–660. PMID: 12778163.
Article9. Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011; 437:169–183. PMID: 21711246.
Article10. Spano D, Zollo M. Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis. 2012; 29:381–395. PMID: 22322279.
Article11. Gao F, Liang B, Reddy ST, Farias-Eisner R, Su X. Role of inflammation-associated microenvironment in tumorigenesis and metastasis. Curr Cancer Drug Targets. 2014; 14:30–45. PMID: 24200082.
Article12. Bellou S, Pentheroudakis G, Murphy C, Fotsis T. Anti-angiogenesis in cancer therapy: Hercules and hydra. Cancer Lett. 2013; 338:219–228. PMID: 23707856.
Article13. Dev S. Studies in sesquiterpenes-XVI: zerumbone, a monocyclic sesquiterpene ketone. Tetrahedron. 1960; 8:171–180.14. Murakami A, Takahashi M, Jiwajinda S, Koshimizu K, Ohigashi H. Identification of zerumbone in Zingiber zerumbet Smith as a potent inhibitor of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation. Biosci Biotechnol Biochem. 1999; 63:1811–1812. PMID: 10586508.15. Kirana C, McIntosh GH, Record IR, Jones GP. Antitumor activity of extract of Zingiber aromaticum and its bioactive sesquiterpenoid zerumbone. Nutr Cancer. 2003; 45:218–225. PMID: 12881017.
Article16. Huang GC, Chien TY, Chen LG, Wang CC. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005; 71:219–224. PMID: 15770541.17. Szabolcs A, Tiszlavicz L, Kaszaki J, Pósa A, Berkó A, Varga IS, Boros I, Szüts V, Lonovics J, Takács T. Zerumbone exerts a beneficial effect on inflammatory parameters of cholecystokinin octapeptide-induced experimental pancreatitis but fails to improve histology. Pancreas. 2007; 35:249–255. PMID: 17895846.
Article18. Kitayama T. Attractive reactivity of a natural product, zerumbone. Biosci Biotechnol Biochem. 2011; 75:199–207. PMID: 21307568.
Article19. Shamoto T, Matsuo Y, Shibata T, Tsuboi K, Nagasaki T, Takahashi H, Funahashi H, Okada Y, Takeyama H. Zerumbone inhibits angiogenesis by blocking NF-κB activity in pancreatic cancer. Pancreas. 2014; 43:396–404. PMID: 24622069.
Article20. Tsuboi K, Matsuo Y, Shamoto T, Shibata T, Koide S, Morimoto M, Guha S, Sung B, Aggarwal BB, Takahashi H, Takeyama H. Zerumbone inhibits tumor angiogenesis via NF-κB in gastric cancer. Oncol Rep. 2014; 31:57–64. PMID: 24220661.
Article21. Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009; 12:267–274. PMID: 19399631.
Article22. Go RS, Owen WG. The rat aortic ring assay for in vitro study of angiogenesis. Methods Mol Med. 2003; 85:59–64. PMID: 12710197.23. Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006; 312:549–560. PMID: 16336962.
Article24. Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009; 8:235–253. PMID: 19247306.
Article25. Woad KJ, Hunter MG, Mann GE, Laird M, Hammond AJ, Robinson RS. Fibroblast growth factor 2 is a key determinant of vascular sprouting during bovine luteal angiogenesis. Reproduction. 2012; 143:35–43. PMID: 21998077.
Article26. Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005; 438:932–936. PMID: 16355210.
Article27. Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. 2014; 41:235–251. PMID: 24787295.
Article28. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011; 146:873–887. PMID: 21925313.
Article29. Ayers M, Fargnoli J, Lewin A, Wu Q, Platero JS. Discovery and validation of biomarkers that respond to treatment with brivanib alaninate, a small-molecule VEGFR-2/FGFR-1 antagonist. Cancer Res. 2007; 67:6899–6906. PMID: 17638901.
Article30. Renhowe PA, Pecchi S, Shafer CM, Machajewski TD, Jazan EM, Taylor C, Antonios-McCrea W, McBride CM, Frazier K, Wiesmann M, Lapointe GR, Feucht PH, Warne RL, Heise CC, Menezes D, Aardalen K, Ye H, He M, Le V, Vora J, Jansen JM, Wernette-Hammond ME, Harris AL. Design, structure-activity relationships and in vivo characterization of 4-amino-3-benzimidazol-2-ylhydroquinolin-2-ones: a novel class of receptor tyrosine kinase inhibitors. J Med Chem. 2009; 52:278–292. PMID: 19113866.
Article31. Bello E, Colella G, Scarlato V, Oliva P, Berndt A, Valbusa G, Serra SC, D'Incalci M, Cavalletti E, Giavazzi R, Damia G, Camboni G. E-3810 is a potent dual inhibitor of VEGFR and FGFR that exerts antitumor activity in multiple preclinical models. Cancer Res. 2011; 71:1396–1405. PMID: 21212416.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Steamed Ginger Extract Exerts Anti-inflammatory Effects in Helicobacter pylori-infected Gastric Epithelial Cells through Inhibition of NF-κB
- Thrombospondin-1 and Inhibition of Tumor Growth
- The Effects of Ginger on Gallbladder Motility in Healthy Male Humans
- Unraveling Stereochemical Structure-Activity Relationships of Sesquiterpene Lactones for Inhibitory Effects on STAT3 Activation
- Effect of Zingiber Officinale Roscoe Extracts on Mice Immune Cell Activation