Korean Circ J.
2019 Apr;49(4):314-325. 10.4070/kcj.2018.0437.
Cortical Bone Derived Stem Cells for Cardiac Wound Healing
- Affiliations
-
- 1Department of Pharmacology, Cardiovascular Research Center, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA. sadia.mohsin@temple.edu
- 2Department of Physiology, Cardiovascular Research Center, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA. srhouser@temple.edu
- KMID: 2441134
- DOI: http://doi.org/10.4070/kcj.2018.0437
Abstract
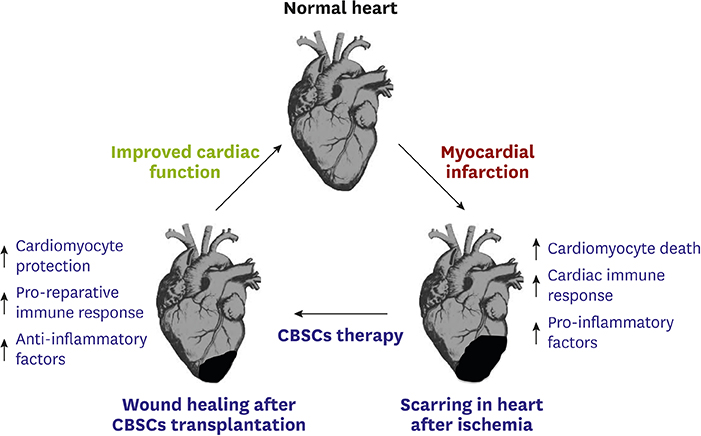
- Ischemic heart disease can lead to myocardial infarction (MI), a major cause of morbidity and mortality worldwide. Adoptive transfer of multiple stem cell types into failing human hearts has demonstrated safety however the beneficial effects in patients with cardiovascular disorders have been modest. Modest improvement in patients with cardiac complications warrants identification of a novel stem cell population that possesses effective reparative properties and improves cardiac function after injury. Recently we have shown in a mouse model and a porcine pre-clinical animal model, that cortical bone derived stem cells (CBSCs) enhance cardiac function after MI and/or ischemia-reperfusion injury. These beneficial effects of allogeneic cell delivery appear to be mediated by paracrine mechanisms rather than by transdifferentiation of injected cells into vessels and/or immature myocytes. This review will discuss role of CBSCs in cardiac wound healing. After having modest beneficial improvement in most of the clinical trials, a critical need is to understand the interaction of the transplanted stem cells with the ischemic cardiac environment. Transplanted stem cells are exposed to pro-inflammatory factors and activated immune cells and fibroblasts, but their interactions remain unknown. We have shown that CBSCs modulate different processes including modulation of the immune response, angiogenesis, and restriction of infarct sizes after cardiac injury. This review will provide information on unique protective signature of CBSCs in rodent/swine animal models for heart repair that should provide basis for developing novel therapies for treating heart failure patients.
MeSH Terms
Figure
Cited by 1 articles
-
Stem Cell and Exosome Therapy in Pulmonary Hypertension
Seyeon Oh, Ji-Hye Jung, Kyung-Jin Ahn, Albert Youngwoo Jang, Kyunghee Byun, Phillip C. Yang, Wook-Jin Chung
Korean Circ J. 2022;52(2):110-122. doi: 10.4070/kcj.2021.0191.
Reference
-
1. Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000; 102:470–479.2. Gerdes AM. Cardiac myocyte remodeling in hypertrophy and progression to failure. J Card Fail. 2002; 8:S264–S268.
Article3. Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016; 119:91–112.4. Golpanian S, Schulman IH, Ebert RF, et al. Concise review: review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl Med. 2016; 5:186–191.
Article5. Loughran JH, Chugh AR, Ismail I, Bolli R. Stem cell therapy: promising treatment in heart failure? Curr Heart Fail Rep. 2013; 10:73–80.
Article6. Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015; 116:1413–1430.
Article7. Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003; 114:763–776.
Article8. Tang XL, Rokosh G, Sanganalmath SK, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010; 121:293–305.
Article9. Rota M, Padin-Iruegas ME, Misao Y, et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008; 103:107–116.
Article10. Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007; 115:896–908.
Article11. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001; 410:701–705.
Article12. Rota M, Kajstura J, Hosoda T, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007; 104:17783–17788.
Article13. Qian L, Huang Y, Spencer CI, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012; 485:593–598.
Article14. Song K, Nam YJ, Luo X, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012; 485:599–604.
Article15. Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012; 379:895–904.
Article16. Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011; 378:1847–1857.
Article17. Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (PROMETHEUS) trial. Circ Res. 2014; 114:1302–1310.18. Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006; 355:1210–1221.
Article19. Yousef M, Schannwell CM, Köstering M, Zeus T, Brehm M, Strauer BE. The BALANCE study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009; 53:2262–2269.20. Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004; 94:92–95.
Article21. Chugh AR, Beache GM, Loughran JH, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012; 126:S54–64.
Article22. Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011; 109:923–940.23. Li TS, Cheng K, Malliaras K, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012; 59:942–953.
Article24. Urbanek K, Rota M, Cascapera S, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005; 97:663–673.
Article25. Tang XL, Rokosh G, Sanganalmath SK, et al. Effects of intracoronary infusion of escalating doses of cardiac stem cells in rats with acute myocardial infarction. Circ Heart Fail. 2015; 8:757–765.
Article26. Wallner M, Duran JM, Mohsin S, et al. Acute catecholamine exposure causes reversible myocyte injury without cardiac regeneration. Circ Res. 2016; 119:865–879.
Article27. Zeng L, Hu Q, Wang X, et al. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007; 115:1866–1875.
Article28. Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006; 113:1451–1463.29. Kucia M, Dawn B, Hunt G, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004; 95:1191–1199.
Article30. Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006; 103:9226–9231.
Article31. Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004; 428:664–668.
Article32. van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014; 509:337–341.
Article33. Smith SC, Zhang X, Zhang X, et al. GDF11 does not rescue aging-related pathological hypertrophy. Circ Res. 2015; 117:926–932.
Article34. Zhu H, Guo ZK, Jiang XX, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010; 5:550–560.
Article35. Duran JM, Makarewich CA, Sharp TE, et al. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res. 2013; 113:539–552.
Article36. Sharp TE 3rd, Schena GJ, Hobby AR, et al. Cortical bone stem cell therapy preserves cardiac structure and function after myocardial infarction. Circ Res. 2017; 121:1263–1278.
Article37. Czubryt MP. Common threads in cardiac fibrosis, infarct scar formation, and wound healing. Fibrogenesis Tissue Repair. 2012; 5:19.
Article38. Aurora AB, Porrello ER, Tan W, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014; 124:1382–1392.
Article39. Lavine KJ, Epelman S, Uchida K, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014; 111:16029–16034.
Article40. Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014; 40:91–104.
Article41. Weirather J, Hofmann UD, Beyersdorf N, et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014; 115:55–67.42. Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015; 15:117–129.
Article43. Ben Nasr M, Vergani A, Avruch J, et al. Co-transplantation of autologous MSCs delays islet allograft rejection and generates a local immunoprivileged site. Acta Diabetol. 2015; 52:917–927.
Article44. Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005; 11:367–368.
Article45. Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006; 20:661–669.
Article46. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008; 103:1204–1219.
Article47. Zsebo KM, Williams DA, Geissler EN, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990; 63:213–224.48. Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004; 109:1543–1549.
Article49. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006; 98:1076–1084.
Article50. Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res. 2016; 118:95–107.
Article51. Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007; 4:Suppl 1. S21–S26.
Article52. Kishore R, Verma SK, Mackie AR, et al. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac miRNA-155 modulates fibrotic response in diabetic hearts. PLoS One. 2013; 8:e60161.
Article53. Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005; 7:223–253.
Article54. Christia P, Bujak M, Gonzalez-Quesada C, et al. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem. 2013; 61:555–570.
Article55. Wessels A, Sedmera D. Developmental anatomy of the heart: a tale of mice and man. Physiol Genomics. 2003; 15:165–176.
Article56. Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol. 1998; 274:H1335–47.57. Piacentino V 3rd, Weber CR, Chen X, et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003; 92:651–658.
Article58. Xu D, Peltz G. Can humanized mice predict drug “behavior” in humans? Annu Rev Pharmacol Toxicol. 2016; 56:323–338.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proliferation of Keratinocytes Induced by Adipose-Derived Stem Cells on a Chitosan Scaffold and Its Role in Wound Healing, a Review
- Human Adipose Mesenchymal Stem Cell-Derived Exosomes: A Key Player in Wound Healing
- Research Advances in the Application of Adipose-Derived Stem Cells Derived Exosomes in Cutaneous Wound Healing
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice