J Korean Med Sci.
2017 Aug;32(8):1327-1336. 10.3346/jkms.2017.32.8.1327.
Identification of Downstream Genes of the mTOR Pathway that Predict Recurrence and Progression in Non-Muscle Invasive High-Grade Urothelial Carcinoma of the Bladder
- Affiliations
-
- 1Department of Urology, Chung-Ang University College of Medicine, Seoul, Korea.
- 2Department of Agricultural Biology, National Academy of Agricultural Science, Rural Development Administration, Jeonju, Korea.
- 3Department of Pathology, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 4Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea. taejlee@cau.ac.kr
- KMID: 2439468
- DOI: http://doi.org/10.3346/jkms.2017.32.8.1327
Abstract
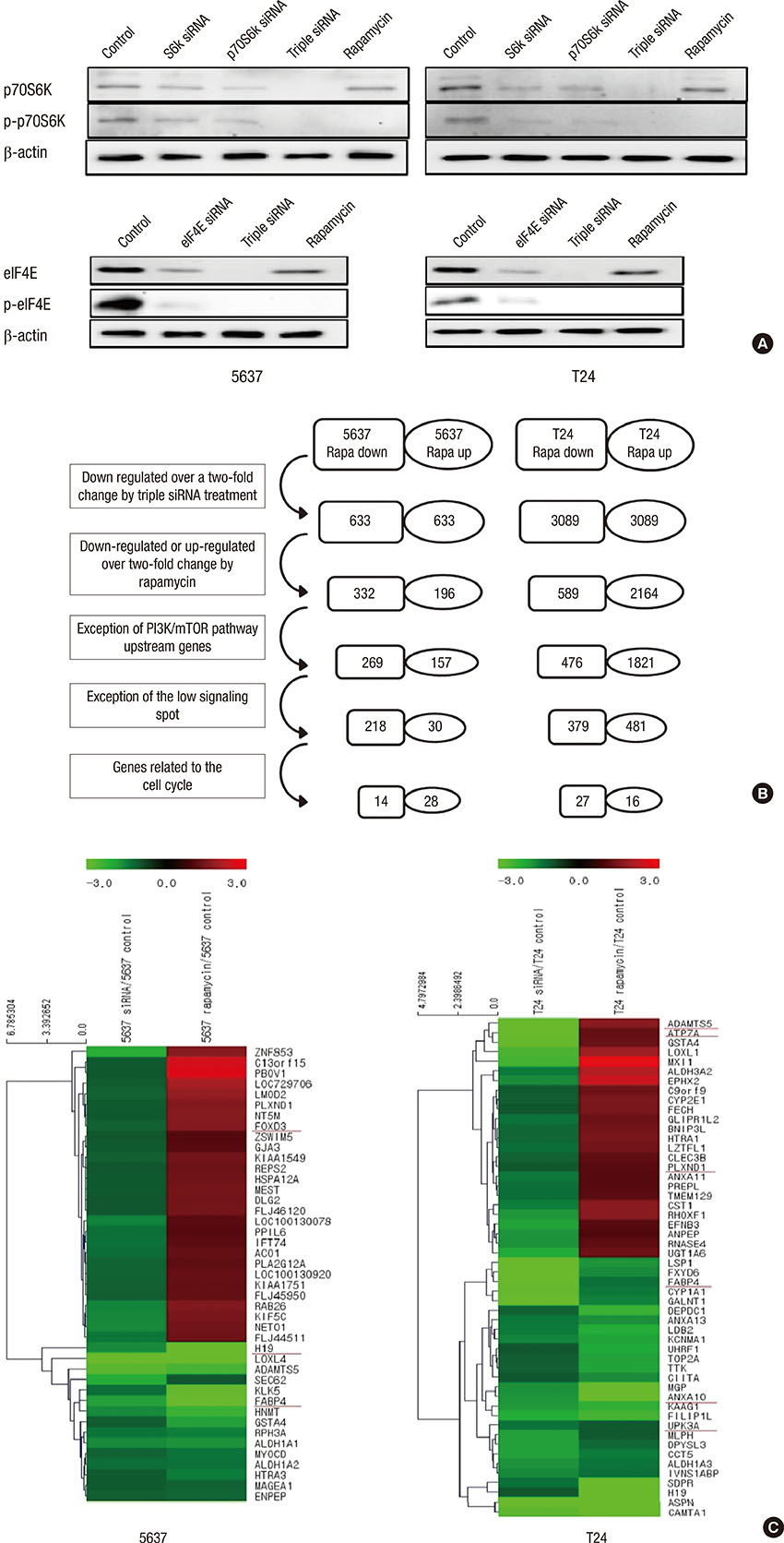
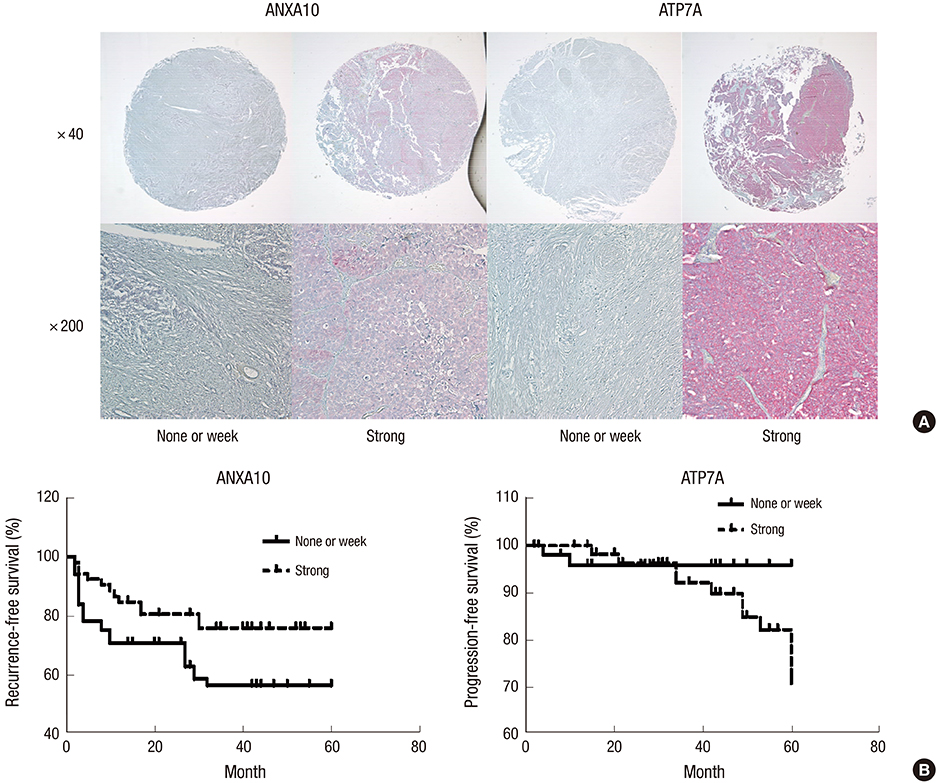
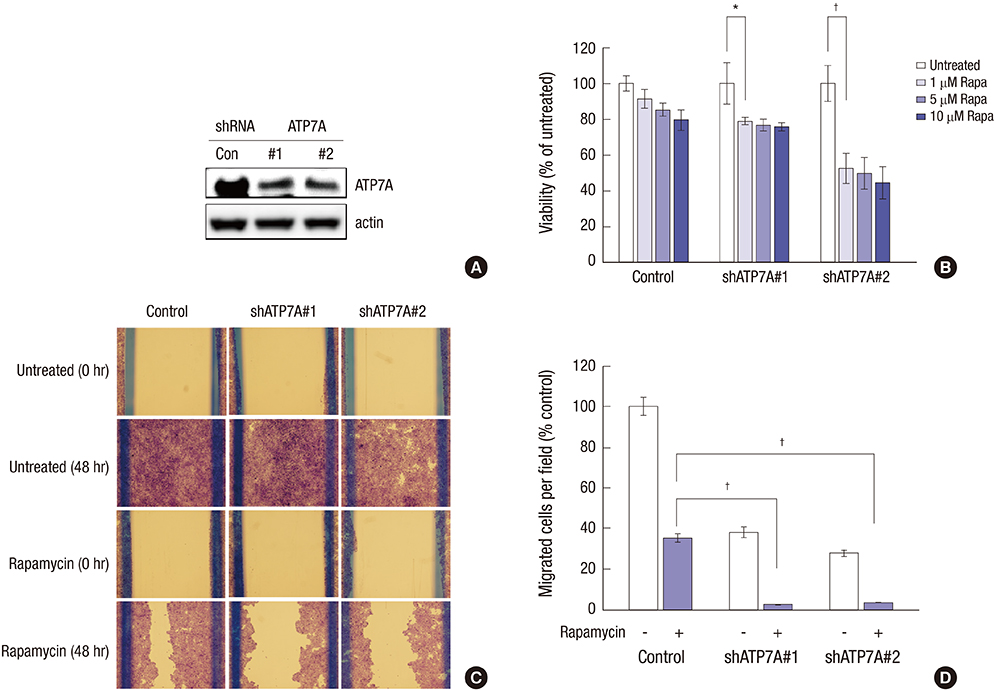
- Microarray analysis was used to investigate the lack of identified mammalian target of rapamycin (mTOR) pathway downstream genes to overcome cross-talk at non-muscle invasive high-grade (HG)-urothelial carcinoma (UC) of the bladder, gene expression patterns, gene ontology, and gene clustering by triple (p70S6K, S6K, and eIF4E) small interfering RNAs (siRNAs) or rapamycin in 5637 and T24 cell lines. We selected mTOR pathway downstream genes that were suppressed by siRNAs more than 2-fold, or were up-regulated or down-regulated by rapamycin more than 2-fold. We validated mTOR downstream genes with immunohistochemistry using a tissue microarray (TMA) of 125 non-muscle invasive HG-UC patients and knockout study to evaluate the synergistic effect with rapamycin. The microarray analysis selected mTOR pathway downstream genes consisting of 4 rapamycin up-regulated genes (FABP4, H19, ANXA10, and UPK3A) and 4 rapamycin down-regulated genes (FOXD3, ATP7A, plexin D1, and ADAMTS5). In the TMA, FABP4, and ATP7A were more expressed at T1 and FOXD3 was at Ta. ANXA10 and ADAMTS5 were more expressed in tumors ≤ 3 cm in diameter. In a multivariate Cox regression model, ANXA10 was a significant predictor of recurrence and ATP7A was a significant predictor of progression in non-muscle invasive HG-UC of the bladder. In an ATP7A knock-out model, rapamycin treatment synergistically inhibited cell viability, wound healing, and invasion ability compared to rapamycin only. Activity of the ANXA10 and ATP7A mTOR pathway downstream genes might predict recurrence and progression in non-muscle invasive HG-UC of the bladder. ATP7A knockout overcomes rapamycin cross-talk.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Letter to the Editor: Bioinformatics Analysis in Downstream Genes of the mTOR Pathway to Predict Recurrence and Progression of Bladder Cancer
Chenyu Li, Hang Liu, Yan Xu
J Korean Med Sci. 2018;33(4):. doi: 10.3346/jkms.2018.33.e31.
Reference
-
1. Garcia JA, Danielpour D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol Cancer Ther. 2008; 7:1347–1354.2. Panwalkar A, Verstovsek S, Giles FJ. Mammalian target of rapamycin inhibition as therapy for hematologic malignancies. Cancer. 2004; 100:657–666.3. Le Tourneau C, Faivre S, Serova M, Raymond E. mTORC1 inhibitors: is temsirolimus in renal cancer telling us how they really work? Br J Cancer. 2008; 99:1197–1203.4. Kwon JK, Kim SJ, Kim JH, Mee Lee KM, Chang IH. Dual inhibition by S6K1 and Elf4E is essential for controlling cellular growth and invasion in bladder cancer. Urol Oncol. 2014; 32:51.e27–51.e35.5. Chi BH, Kim SJ, Seo HK, Seo HH, Lee SJ, Kwon JK, Lee TJ, Chang IH. P70S6K and Elf4E dual inhibition is essential to control bladder tumor growth and progression in orthotopic mouse non-muscle invasive bladder tumor model. J Korean Med Sci. 2015; 30:308–316.6. Kim SJ, Kim JH, Jung HS, Lee TJ, Lee KM, Chang IH. Phosphorylated p70S6K in noninvasive low-grade urothelial carcinoma of the bladder: correlation with tumor recurrence. Asian J Androl. 2014; 16:611–617.7. Milowsky MI, Iyer G, Regazzi AM, Al-Ahmadie H, Gerst SR, Ostrovnaya I, Gellert LL, Kaplan R, Garcia-Grossman IR, Pendse D, et al. Phase II study of everolimus in metastatic urothelial cancer. BJU Int. 2013; 112:462–470.8. Seront E, Rottey S, Sautois B, Kerger J, D’Hondt LA, Verschaeve V, Canon JL, Dopchie C, Vandenbulcke JM, Whenham N, et al. Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: clinical activity, molecular response, and biomarkers. Ann Oncol. 2012; 23:2663–2670.9. Detre S, Saclani Jotti G, Dowsett MA. “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995; 48:876–878.10. Heinonen H, Nieminen A, Saarela M, Kallioniemi A, Klefström J, Hautaniemi S, Monni O. Deciphering downstream gene targets of PI3K/mTOR/p70S6K pathway in breast cancer. BMC Genomics. 2008; 9:348.11. Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002; 82:331–371.12. Hayes MJ, Moss SE. Annexins and disease. Biochem Biophys Res Commun. 2004; 322:1166–1170.13. Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005; 6:449–461.14. Munksgaard PP, Mansilla F, Brems Eskildsen AS, Fristrup N, Birkenkamp-Demtröder K, Ulhøi BP, Borre M, Agerbæk M, Hermann GG, Orntoft TF, et al. Low ANXA10 expression is associated with disease aggressiveness in bladder cancer. Br J Cancer. 2011; 105:1379–1387.15. Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007; 33:9–23.16. Safaei R. Role of copper transporters in the uptake and efflux of platinum containing drugs. Cancer Lett. 2006; 234:34–39.17. Choi SY, Ryu JH, Chang IH, Kim TH, Myung SC, Moon YT, Kim KD, Kim JW. Predicting recurrence and progression of non-muscle-invasive bladder cancer in Korean patients: a comparison of the EORTC and CUETO models. Korean J Urol. 2014; 55:643–649.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NBR1 and KIF14 Downstream of the Mammarian Target of Rapamycin Pathway Predict Recurrence in Nonmuscle Invasive Low Grade Urothelial Carcinoma of the Bladder
- Letter to the Editor: Bioinformatics Analysis in Downstream Genes of the mTOR Pathway to Predict Recurrence and Progression of Bladder Cancer
- The Author's Response: Bioinformatics Analysis in Downstream Genes of the mTOR Pathway to Predict Recurrence and Progression of Bladder Cancer
- Autophagy and urothelial carcinoma of the bladder: A review
- Significance of tumor-associated neutrophils, lymphocytes, and neutrophil-to-lymphocyte ratio in non-invasive and invasive bladder urothelial carcinoma