Ann Lab Med.
2019 Jul;39(4):388-395. 10.3343/alm.2019.39.4.388.
Enumeration of CD34-positive Stem Cells Using the ADAMII Image-based Fluorescence Cell Counter
- Affiliations
-
- 1Laboratory Development and Evaluation Center, College of Medicine, The Catholic University of Korea, Seoul, Korea. yonggoo@catholic.ac.kr, microkim@catholic.ac.kr
- 2Department of Biomedicine & Health Sciences, Graduate School, The Catholic University of Korea, Seoul, Korea.
- 3Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4NanoEntek, Seoul, Korea.
- KMID: 2438886
- DOI: http://doi.org/10.3343/alm.2019.39.4.388
Abstract
- BACKGROUND
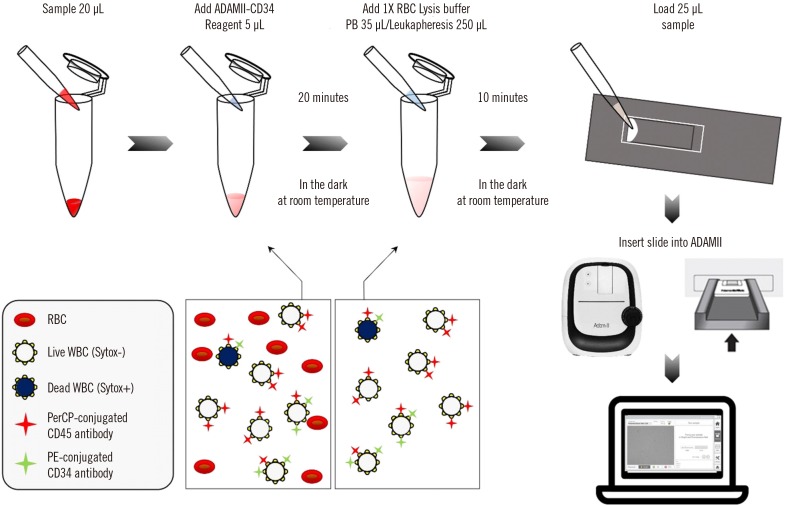
It is very important to accurately enumerate CD34-positive (CD34+) cells for successful hematopoietic stem cell transplantation (HSCT). We evaluated the ability of the newly developed image based-immunofluorescence cell counter ADAMII (NanoEntek, Seoul, Korea) to enumerate CD34+ cells, which was improved through simultaneous CD45 analysis.
METHODS
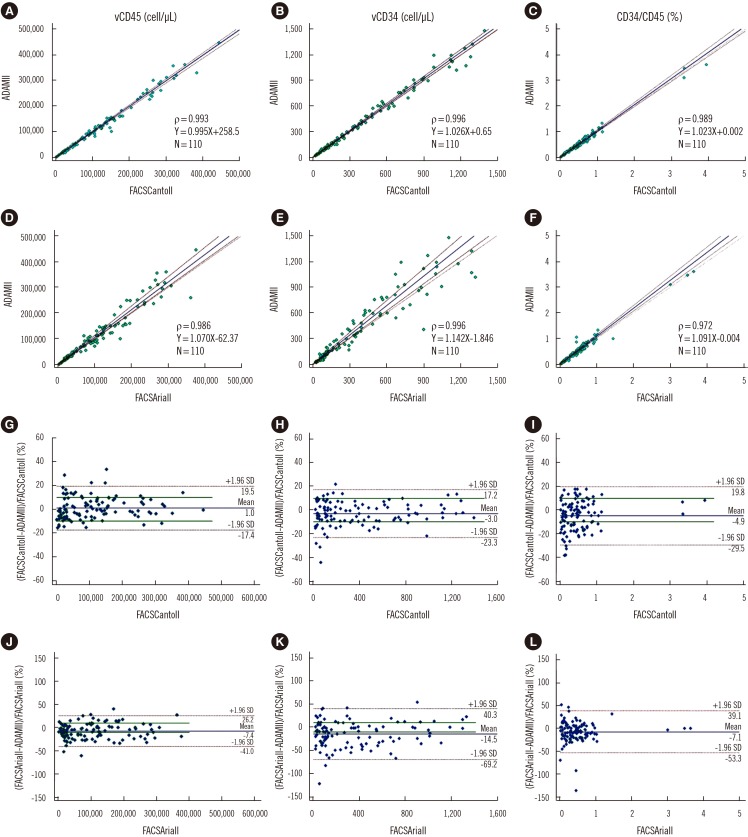
We enumerated CD34+ cells with ADAMII using 19 peripheral blood (PB) and 91 leukapheresis samples from HSCT donors. Analytical performance, including precision and linearity, was analyzed, and sample stability during storage was evaluated. Viable CD34+ cell count (vCD34) and viable CD45+ cell count (vCD45) and the percentage of viable CD34+ cells among viable CD45+ cells (CD34/CD45) as measured by ADAMII were compared with the corresponding values from two flow cytometry assays, using regression analysis.
RESULTS
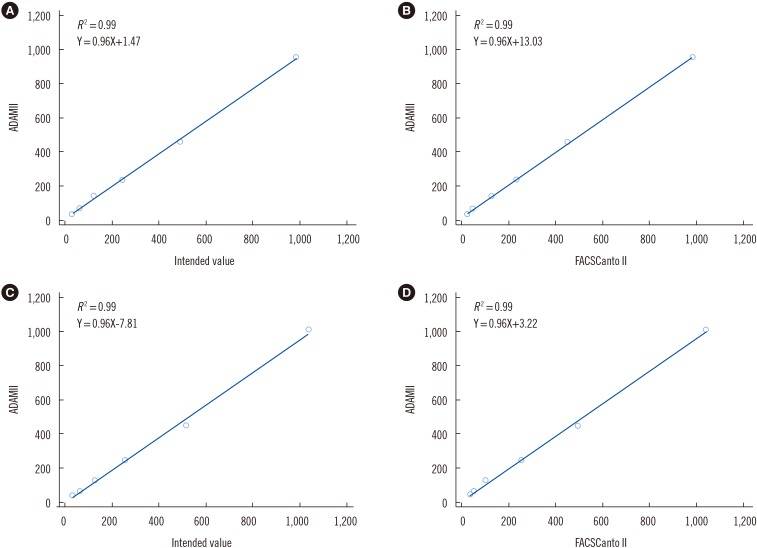
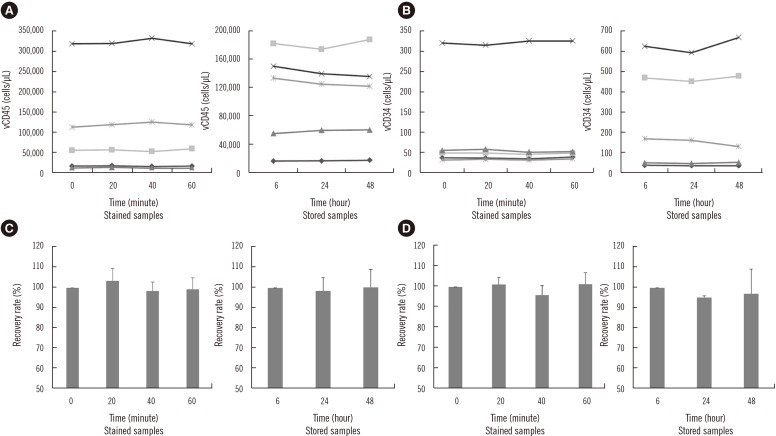
ADAMII demonstrated acceptable precision, as CV values of vCD34 from six samples with different counts were all < 10% (range: 3.49-9.51%). CV values of the vCD45 and CD34/45 ranged from 4.03% to 9.67% and from 2.48% to 10.07%, respectively. The linearity of vCD34 showed an excellent R 2 value (0.99) when analyzed using the intended count and flow cytometry data. The ADAMII and two flow cytometry-based assays generated very similar data for the PB and leukapheresis samples.
CONCLUSIONS
ADAMII demonstrated excellent performance for use as a routine clinical assay in terms of CD34+ cell enumeration from PB and leukapheresis samples. Moreover, it could be used as a point-of-care-test for determining mobilization time and predicting an adequate apheresis stem cell product.
MeSH Terms
Figure
Reference
-
1. Bensinger WI, Clift RA, Anasetti C, Appelbaum FA, Demirer T, Rowley S, et al. Transplantation of allogeneic peripheral blood stem cells mobilized by recombinant human granulocyte colony stimulating factor. Stem Cells. 1996; 14:90–105. PMID: 8820955.2. Schmitz N, Dreger P, Suttorp M, Rohwedder EB, Haferlach T, Löffler H, et al. Primary transplantation of allogeneic peripheral blood progenitor cells mobilized by filgrastim (granulocyte colony-stimulating factor). Blood. 1995; 85:1666–1672. PMID: 7534141.3. Bender JG, To LB, Williams S, Schwartzberg LS. Defining a therapeutic dose of peripheral blood stem cells. J Hematother. 1992; 1:329–341. PMID: 1285382.4. Lie AK, To LB. Peripheral blood stem cells: transplantation and beyond. Oncologist. 1997; 2:40–49. PMID: 10388028.5. Massin F, Huili C, Decot V, Stoltz JF, Bensoussan D, Latger-Cannard V. Validation of a single-platform method for hematopoietic CD34+ stem cells enumeration according to accreditation procedure. Biomed Mater Eng. 2015; 25(S1):27–39. PMID: 25538053.6. Allan DS, Keeney M, Howson-Jan K, Popma J, Weir K, Bhatia M, et al. Number of viable CD34(+) cells reinfused predicts engraftment in autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002; 29:967–972. PMID: 12098064.7. Noga SJ, Vogelsang GB, Miller SC, Meusel S, Loper K, Case R, et al. Using point-of-care CD34 enumeration to optimize PBSC collection conditions. Cytotherapy. 2001; 3:11–18. PMID: 12028839.8. Gigant C, Latger-Cannard V, Bensoussan D, Feugier P, Bordigoni P, Stoltz JF. Quantitative expression of adhesion molecules on granulocyte colony-stimulating factor-mobilized peripheral blood, bone marrow, and cord blood CD34+ cells. J Hematother Stem Cell Res. 2001; 10:807–814. PMID: 11798507.9. Barnett D, Janossy G, Lubenko A, Matutes E, Newland A, Reilly JT. Guideline for the flow cytometric enumeration of CD34+ haematopoietic stem cells. Prepared by the CD34+ haematopoietic stem cell working party. General Haematology Task Force of the British Committee for Standards in Haematology. Clin Lab Haematol. 1999; 21:301–308. PMID: 10646072.10. Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR. Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. International Society of Hematotherapy and Graft Engineering. Cytometry. 1998; 34:61–70. PMID: 9579602.11. Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996; 5:213–226. PMID: 8817388.12. Sutherland DR, Nayyar R, Acton E, Giftakis A, Dean S, Mosiman VL. Comparison of two single-platform ISHAGE-based CD34 enumeration protocols on BD FACSCalibur and FACSCanto flow cytometers. Cytotherapy. 2009; 11:595–605. PMID: 19513900.13. CLSI. Evaluation of precision performance of quantitative measurement methods, CLSI EP5-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2004.14. CLSI. Evaluation of the linearity of quantitative measurement procedures: a statistical approach CLSI EP6-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.15. Dietz LJ, Dubrow RS, Manian BS, Sizto NL. Volumetric capillary cytometry: a new method for absolute cell enumeration. Cytometry. 1996; 23:177–186. PMID: 8974863.16. Olivero S, Alario T, Ladaique P, Haccoun M, Viens P, Blaise D, et al. CD34+ cell enumeration in peripheral blood and apheresis samples, using two laboratory diagnostic kits or an institutional protocol. Bone Marrow Transplant. 1999; 23:387–394. PMID: 10100583.17. Chapple P, Prince HM, Wall D, Filshie R, Haylock D, Quinn M, et al. Comparison of three methods of CD34+ cell enumeration in peripheral blood: dual-platform ISHAGE protocol versus single-platform, versus microvolume fluorimetry. Cytotherapy. 2000; 2:371–376. PMID: 12044229.18. Cho MO, Kim S, Lee JY, Oh JH, Kim JY, Bong SR, et al. Performance evaluation of an automated image-based fluorescence CD4+ cell analyzer. Technol Health Care. 2018; 26:867–871. PMID: 30040773.19. Lane TA, Bashey A, Carrier E, Holman P, Castro J, Mullen M, et al. Improving the efficiency of PBPC collection by pre-apheresis peripheral blood and mid-apheresis product measurements of CD34 cells. Cytotherapy. 2004; 6:318–327. PMID: 16146884.20. Sidhu RS, Orsini E Jr, Giller R, Quinones R, Foreman NK, Thompson H, et al. Midpoint CD34 measurement as a predictor of PBPC product yield in pediatric patients undergoing high-dose chemotherapy. J Clin Apher. 2006; 21:165–168. PMID: 16425193.21. Chepovetsky J, Choo Yoon S, Blouin AG, Tindle S, Bertinelli A, Nash E, Wu DW. Roles of Peripheral Blood CD34+ Cell Count and Midpoint Collection CD34+ Cell Yield for Peripheral Blood Stem Cell Collections from Autologous Patients Mobilized by G-CSF and Plerixafor. N Am J Med Sci (Boston). 2013; 6:63–70.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantitation of CD34 Positive Hematopoietic Stem Cells in Cord Blood by Flow Cytometric Analysis: Comparison of 3 Color Method (ProCOUNTTM) and 2 Color Method
- Clinical Applicability of Newly Developed Image-based Cell Counter for Counting CD34+ Cells: Comparison with Flow Cytometric Analysis
- Cutoff Value of PB CD34+ Cells for Optimization of PBSC Collection
- Significance of the Peripheral Blood CD34+ Cell Count by Stem-Kit in Peripheral Blood Stem Cell Collection
- The New Sysmex XN-2000 Automated Blood Cell Analyzer More Accurately Measures the Absolute Number and the Proportion of Hematopoietic Stem and Progenitor Cells Than XE-2100 When Compared to Flow Cytometric Enumeration of CD34+ Cells