J Periodontal Implant Sci.
2012 Dec;42(6):248-255.
Surface characteristics and osteoblastic cell response of alkali-and heat-treated titanium-8tantalum-3niobium alloy
- Affiliations
-
- 1Department of Periodontology, Dental Research Institute, Chonnam National University School of Dentistry, Gwangju, Korea. youngjun@chonnam.ac.kr
- 2Department of Prosthodontics, Chonnam National University School of Dentistry, Gwangju, Korea.
Abstract
- PURPOSE
The aim of the present study was to evaluate the biological response of alkali- and heat-treated titanium-8tantalum-3niobium surfaces by cell proliferation and alkaline phosphatase (ALP) activity analysis.
METHODS
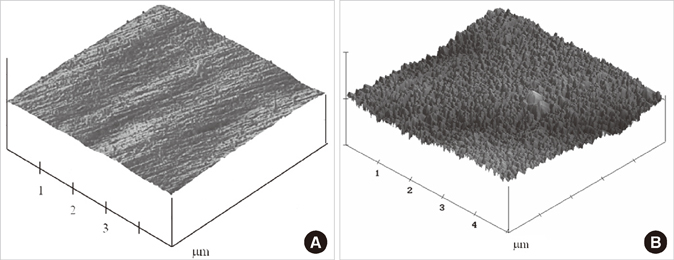
Commercial pure titanium (group cp-Ti) and alkali- and heat-treated titanium-8tantalum-3niobium (group AHT) disks were prepared. The surface properties were evaluated using scanning electron microscopy, energy dispersed spectroscopy and X-ray photoelectron spectroscopy (XPS). The surface roughness was evaluated by atomic force microscopy and a profilometer. The contact angle and surface energy were also analyzed. The biological response of fetal rat calvarial cells on group AHT was assessed by cell proliferation and ALP activity.
RESULTS
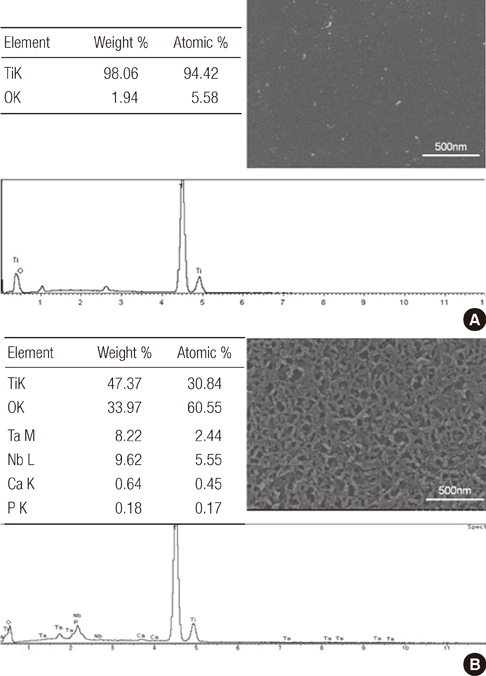
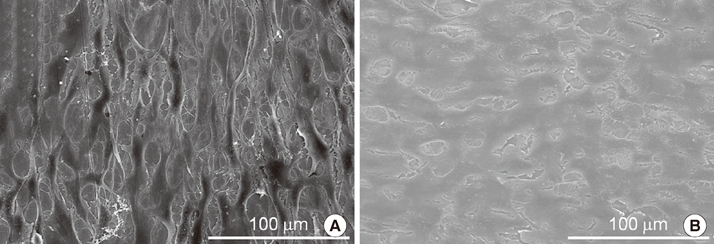
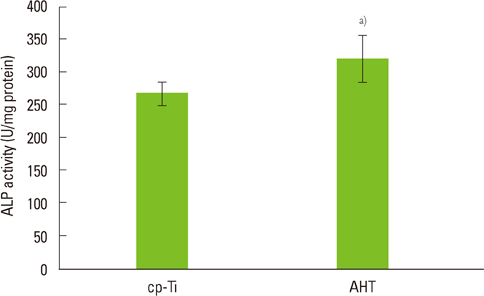
Group AHT showed a flake-like morphology microprofile and dense structure. XPS analysis of group AHT showed an increased amount of oxygen in the basic hydroxyl residue of titanium hydroxide groups compared with group cp-Ti. The surface roughness (Ra) measured by a profilometer showed no significant difference (P>0.05). Group AHT showed a lower contact angle and higher surface energy than group cp-Ti. Cell proliferation on group AHT surfaces was significantly higher than on group cp-Ti surfaces (P<0.05). In comparison to group cp-Ti, group AHT enhanced ALP activity (P<0.05).
CONCLUSIONS
These results suggest that group AHT stimulates osteoblast differentiation.
MeSH Terms
Figure
Reference
-
1. Park JB, Lakes RS. Biomaterials: an introduction. 1992. 2nd ed. New York: Kluwer Academic Publishers.2. Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res. 1998. 40:1–11.
Article3. Li D, Ferguson SJ, Beutler T, Cochran DL, Sittig C, Hirt HP, et al. Biomechanical comparison of the sandblasted and acid-etched and the machined and acid-etched titanium surface for dental implants. J Biomed Mater Res. 2002. 60:325–332.
Article4. Kokubo T, Miyaji F, Kim HM, Nakamura T. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J Am Ceram Soc. 1996. 79:1127–1129.
Article5. Shi S, Kirk M, Kahn AJ. The role of type I collagen in the regulation of the osteoblast phenotype. J Bone Miner Res. 1996. 11:1139–1145.
Article6. Sisk MA, Lohmann CH, Cochran DL, Sylvia VL, Simpson JP, Dean DD, et al. Inhibition of cyclooxygenase by indomethacin modulates osteoblast response to titanium surface roughness in a time-dependent manner. Clin Oral Implants Res. 2001. 12:52–61.
Article7. de Groot K, Wolke JG, Jansen JA. Calcium phosphate coatings for medical implants. Proc Inst Mech Eng H. 1998. 212:137–147.
Article8. Nishiguchi S, Nakamura T, Kobayashi M, Kim HM, Miyaji F, Kokubo T. The effect of heat treatment on bone-bonding ability of alkali-treated titanium. Biomaterials. 1999. 20:491–500.
Article9. Nishiguchi S, Kato H, Neo M, Oka M, Kim HM, Kokubo T, et al. Alkali- and heat-treated porous titanium for orthopedic implants. J Biomed Mater Res. 2001. 54:198–208.
Article10. Nishiguchi S, Fujibayashi S, Kim HM, Kokubo T, Nakamura T. Biology of alkali- and heat-treated titanium implants. J Biomed Mater Res A. 2003. 67:26–35.
Article11. Green J, Schotland S, Stauber DJ, Kleeman CR, Clemens TL. Cell-matrix interaction in bone: type I collagen modulates signal transduction in osteoblast-like cells. Am J Physiol. 1995. 268(5 Pt 1):C1090–C1103.
Article12. McCarthy TL, Centrella M, Canalis E. Further biochemical and molecular characterization of primary rat parietal bone cell cultures. J Bone Miner Res. 1988. 3:401–408.
Article13. Healy KE, Ducheyne P. Hydration and preferential molecular adsorption on titanium in vitro. Biomaterials. 1992. 13:553–561.
Article14. Luben RA, Wong GL, Cohn DV. Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology. 1976. 99:526–534.
Article15. Lincks J, Boyan BD, Blanchard CR, Lohmann CH, Liu Y, Cochran DL, et al. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998. 19:2219–2232.
Article16. Kawahara H, Soeda Y, Niwa K, Takahashi M, Kawahara D, Araki N. In vitro study on bone formation and surface topography from the standpoint of biomechanics. J Mater Sci Mater Med. 2004. 15:1297–1307.
Article17. Berglundh T, Gotfredsen K, Zitzmann NU, Lang NP, Lindhe J. Spontaneous progression of ligature induced peri-implantitis at implants with different surface roughness: an experimental study in dogs. Clin Oral Implants Res. 2007. 18:655–661.
Article18. Abrahamsson I, Berglundh T, Linder E, Lang NP, Lindhe J. Early bone formation adjacent to rough and turned endosseous implant surfaces. An experimental study in the dog. Clin Oral Implants Res. 2004. 15:381–392.
Article19. Pesskova V, Kubies D, Hulejova H, Himmlova L. The influence of implant surface properties on cell adhesion and proliferation. J Mater Sci Mater Med. 2007. 18:465–473.
Article20. Sultana R, Kon M, Hirakata LM, Fujihara E, Asaoka K, Ichikawa T. Surface modification of titanium with hydrothermal treatment at high pressure. Dent Mater J. 2006. 25:470–479.
Article21. Jonasova L, Muller FA, Helebrant A, Strnad J, Greil P. Biomimetic apatite formation on chemically treated titanium. Biomaterials. 2004. 25:1187–1194.
Article22. Kim HM, Miyaji F, Kokubo T, Nishiguchi S, Nakamura T. Graded surface structure of bioactive titanium prepared by chemical treatment. J Biomed Mater Res. 1999. 45:100–107.
Article23. Kim HM, Miyaji F, Kokubo T, Nakamura T. Effect of heat treatment on apatite-forming ability of Ti metal induced by alkali treatment. J Mater Sci Mater Med. 1997. 8:341–347.24. Tamilselvi S, Raghavendran HB, Srinivasan P, Rajendran N. In vitro and in vivo studies of alkali- and heat-treated Ti-6Al-7Nb and Ti-5Al-2Nb-1Ta alloys for orthopedic implants. J Biomed Mater Res A. 2009. 90:380–386.25. Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005. 74:49–58.
Article26. Lian JB, Stein GS. The developmental stages of osteoblast growth and differentiation exhibit selective responses of genes to growth factors (TGF beta 1) and hormones (vitamin D and glucocorticoids). J Oral Implantol. 1993. 19:95–105.27. Genge BR, Sauer GR, Wu LN, McLean FM, Wuthier RE. Correlation between loss of alkaline phosphatase activity and accumulation of calcium during matrix vesicle-mediated mineralization. J Biol Chem. 1988. 263:18513–18519.
Article28. Chosa N, Taira M, Saitoh S, Sato N, Araki Y. Characterization of apatite formed on alkaline-heat-treated Ti. J Dent Res. 2004. 83:465–469.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biological response of primary rat calvarial cell by surface treatment of Ti-8Ta-8Nb alloy
- Surface characteristics and stability of implants treated with alkali and heat
- A histomorphometric study on the effect of surface treatment on the osseointegration
- On the effects of the characteristics of the titanium oxide to the osteoblast cell culture
- The effect of implant surface treated by anodizing on proliferation of the rat osteoblast