Yonsei Med J.
2019 Feb;60(2):148-157. 10.3349/ymj.2019.60.2.148.
MiR-182-5p Knockdown Targeting PTEN Inhibits Cell Proliferation and Invasion of Breast Cancer Cells
- Affiliations
-
- 1Department of Breast Surgery, The Third Hospital Affiliated to Qiqihar Medical College, Qiqihar, China.
- 2Department of Breast Surgery, Jinan Zhangqiu District Hospital of Traditional Chinese Medicine, Zhangqi District, Jinan, China.
- 3Department of General Surgery, Sixth People's Hospital of Ji'nan City, Jinan, China.
- 4Department of Breast Surgery, Sixth People's Hospital of Ji'nan City, Jinan, China. shdlkajhdk750@126.com
- KMID: 2431631
- DOI: http://doi.org/10.3349/ymj.2019.60.2.148
Abstract
- PURPOSE
Breast cancer (BC) is one of the most common malignant tumors, affecting a significant number of women worldwide. MicroRNAs (miRNAs) have been reported to play important roles in tumorigenesis. The aim of this study was to determine the roles of miR-182-5p in BC progression.
MATERIALS AND METHODS
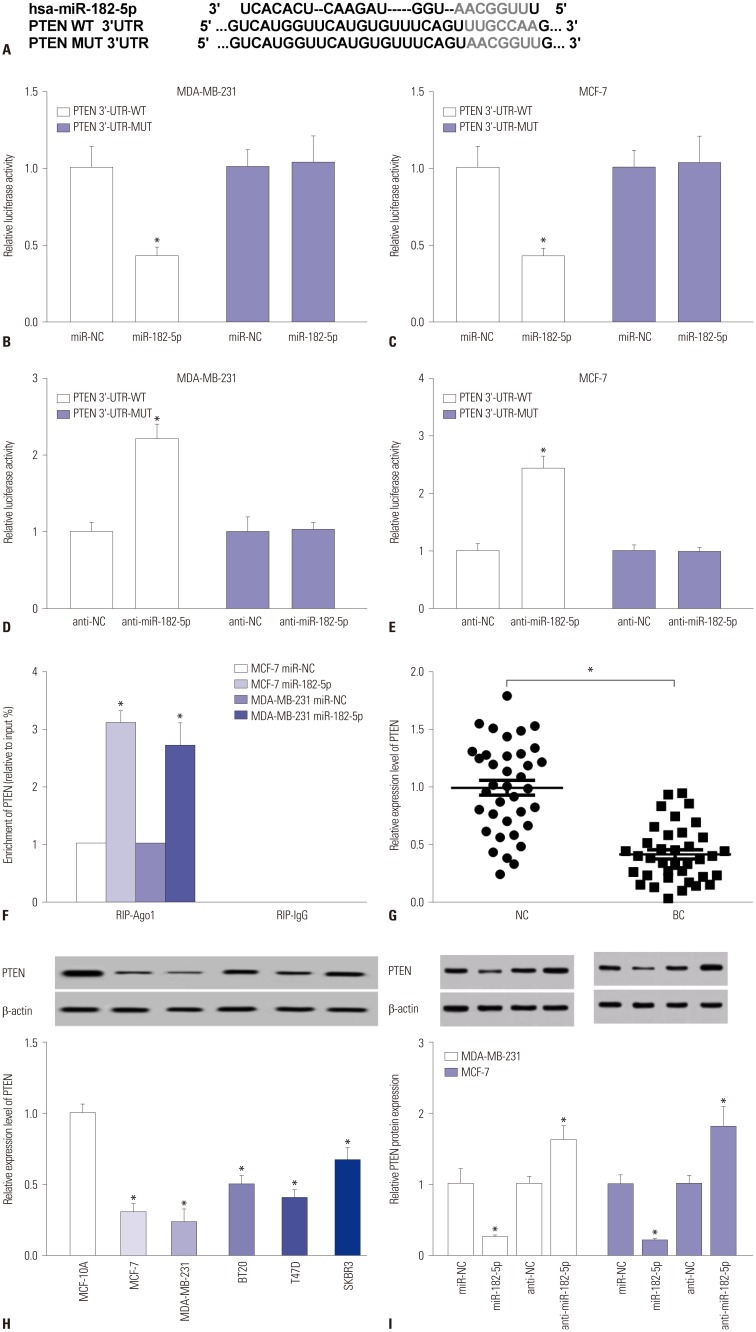
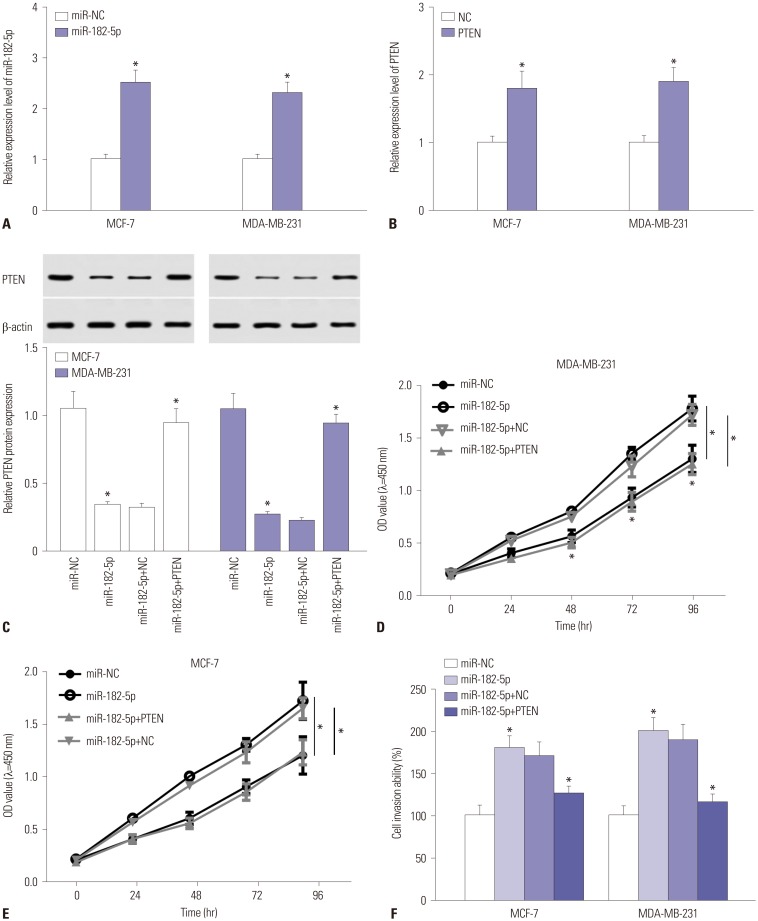
The expressions of miR-182-5p and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) were measured in BC tissues and cells by quantitative real-time polymerase chain reaction or Western blot. Cell proliferation and invasion were detected by cell counting kit-8 assay and trans-well assay, respectively. The interaction between miR-182-5p and PTEN was probed by bioinformatics analysis, luciferase activity, and RNA immunoprecipitation. A murine xenograft model was established to investigate the role of miR-182-5p in BC progression in vivo.
RESULTS
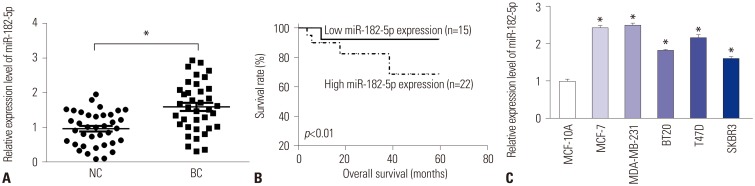
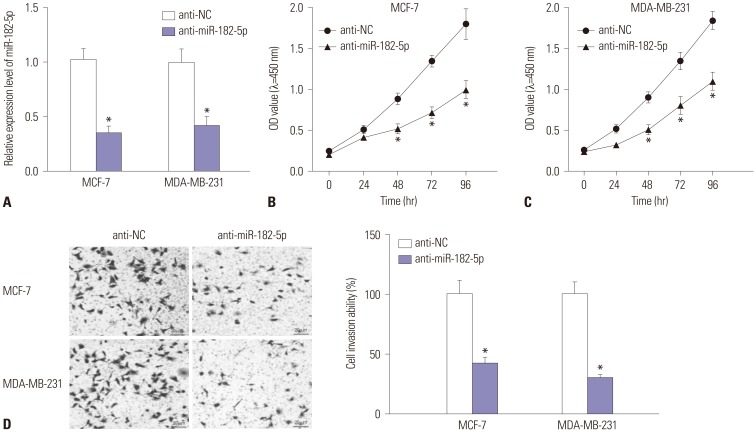
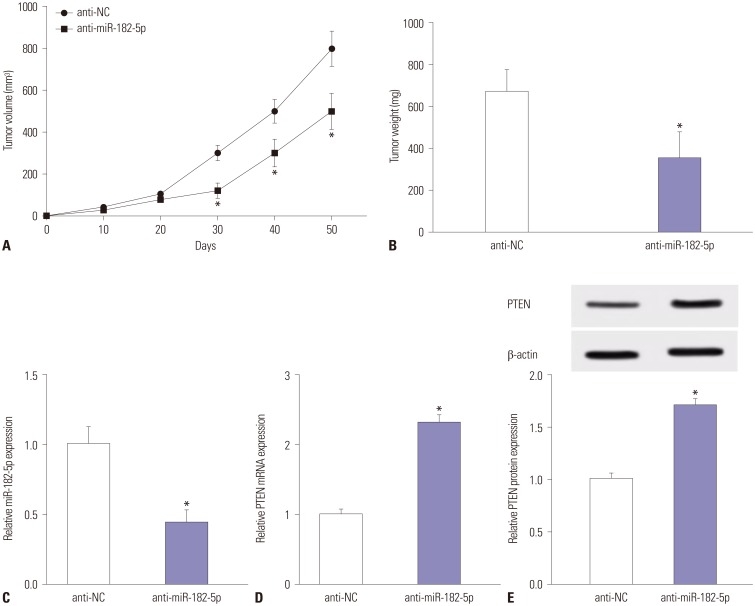
An abundance of miR-182-5p was noted in BC tissues and cells. High expression of miR-182-5p was associated with poor survival. Abrogation of miR-182-5p inhibited cell proliferation and invasion in BC cells. Interestingly, PTEN was indicated as a target of miR-182-5p, and its restoration reversed miR-182-5p-mediated promotion of proliferation and invasion of BC cells. Moreover, depletion of miR-182-5p suppressed tumor growth via up-regulating PTEN expression in the murine xenograft model.
CONCLUSION
MiR-182-5p exhaustion blocked cell proliferation and invasion by regulating PTEN expression, providing a novel therapeutic avenue for treatment of BC.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30. PMID: 29313949.
Article2. Varghese F, Wong J. Breast cancer in the elderly. Surg Clin North Am. 2018; 98:819–833. PMID: 30005776.
Article3. Greenwood HI, Dodelzon K, Katzen JT. Impact of advancing technology on diagnosis and treatment of breast cancer. Surg Clin North Am. 2018; 98:703–724. PMID: 30005769.
Article4. Mandujano-Tinoco EA, García-Venzor A, Melendez-Zajgla J, Maldonado V. New emerging roles of microRNAs in breast cancer. Breast Cancer Res Treat. 2018; 171:247–259. PMID: 29948402.
Article5. O'Bryan S, Dong S, Mathis JM, Alahari SK. The roles of oncogenic miRNAs and their therapeutic importance in breast cancer. Eur J Cancer. 2017; 72:1–11. PMID: 27997852.6. Zhang N, Zhang H, Liu Y, Su P, Zhang J, Wang X, et al. SREBP1, targeted by miR-18a-5p, modulates epithelial-mesenchymal transition in breast cancer via forming a co-repressor complex with Snail and HDAC1/2. Cell Death Differ. 2018; 7. 09. [Epub]. DOI: 10.1038/s41418-018-0158-8.
Article7. Yin C, Zhang G, Sun R, Pan X, Wang X, Li H, et al. miR1855p inhibits Factin polymerization and reverses epithelial mesenchymal transition of human breast cancer cells by modulating RAGE. Mol Med Rep. 2018; 18:2621–2630. PMID: 30015912.
Article8. Li C, Zhang J, Ma Z, Zhang F, Yu W. miR-19b serves as a prognostic biomarker of breast cancer and promotes tumor progression through PI3K/AKT signaling pathway. Onco Targets Ther. 2018; 11:4087–4095. PMID: 30038508.
Article9. Croset M, Pantano F, Kan C, Bonnelye E, Descotes F, Alix-Panabières C, et al. miRNA-30 family members inhibit breast cancer invasion, osteomimicry, and bone destruction by directly targeting multiple bone metastasis-associated genes. Cancer Res. 2018; 78:5259–5273. PMID: 30042152.
Article10. Guo J, Liu C, Wang W, Liu Y, He H, Chen C, et al. Identification of serum miR-1915-3p and miR-455-3p as biomarkers for breast cancer. PLoS One. 2018; 13:e0200716. PMID: 30048472.
Article11. Li Y, Chen S, Shan Z, Bi L, Yu S, Li Y, et al. miR-182-5p improves the viability, mitosis, migration, and invasion ability of human gastric cancer cells by down-regulating RAB27A. Biosci Rep. 2017; 37:BSR20170136. PMID: 28546229.
Article12. Hirata H, Ueno K, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, et al. MicroRNA-182-5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. PLoS One. 2013; 8:e55502. PMID: 23383207.
Article13. Zhang K, Wang YW, Wang YY, Song Y, Zhu J, Si PC, et al. Identification of microRNA biomarkers in the blood of breast cancer patients based on microRNA profiling. Gene. 2017; 619:10–20. PMID: 28359916.
Article14. Sharifi M, Moridnia A. Apoptosis-inducing and antiproliferative effect by inhibition of miR-182-5p through the regulation of CASP9 expression in human breast cancer. Cancer Gene Ther. 2017; 24:75–82. PMID: 28084318.
Article15. Chen CY, Chen J, He L, Stiles BL. PTEN: tumor suppressor and metabolic regulator. Front Endocrinol (Lausanne). 2018; 9:338. PMID: 30038596.
Article16. Chai C, Wu H, Wang B, Eisenstat DD, Leng RP. MicroRNA-498 promotes proliferation and migration by targeting the tumor suppressor PTEN in breast cancer cells. Carcinogenesis. 2018; 39:1185–1196. PMID: 29985991.
Article17. Xu W, Wang W. MicroRNA1425p modulates breast cancer cell proliferation and apoptosis by targeting phosphatase and tensin homolog. Mol Med Rep. 2018; 17:7529–7536. PMID: 29620260.
Article18. Bertoli G, Cava C, Castiglioni I. MicroRNAs: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015; 5:1122–1143. PMID: 26199650.
Article19. Krishnan K, Steptoe AL, Martin HC, Wani S, Nones K, Waddell N, et al. MicroRNA-182-5p targets a network of genes involved in DNA repair. RNA. 2013; 19:230–242. PMID: 23249749.
Article20. Bao S, Wang X, Wang Z, Yang J, Liu F, Yin C. MicroRNA-30 mediates cell invasion and metastasis in breast cancer. Biochem Cell Biol. 2018; 6. 12. [Epub]. DOI: 10.1139/bcb-2018-0032.
Article21. Eastlack SC, Dong S, Ivan C, Alahari SK. Suppression of PDHX by microRNA-27b deregulates cell metabolism and promotes growth in breast cancer. Mol Cancer. 2018; 17:100. PMID: 30012170.
Article22. Zhang T, Jiang K, Zhu X, Zhao G, Wu H, Deng G, et al. miR-433 inhibits breast cancer cell growth via the MAPK signaling pathway by targeting Rap1a. Int J Biol Sci. 2018; 14:622–632. PMID: 29904277.
Article23. Cao MQ, You AB, Zhu XD, Zhang W, Zhang YY, Zhang SZ, et al. miR-182-5p promotes hepatocellular carcinoma progression by repressing FOXO3a. J Hematol Oncol. 2018; 11:12. PMID: 29361949.
Article24. Rousset-Jablonski C, Gompel A. Screening for familial cancer risk: focus on breast cancer. Maturitas. 2017; 105:69–77. PMID: 28818315.
Article25. Dai X, Fang M, Li S, Yan Y, Zhong Y, Du B. miR-21 is involved in transforming growth factor β1-induced chemoresistance and invasion by targeting PTEN in breast cancer. Oncol Lett. 2017; 14:6929–6936. PMID: 29151919.
Article26. Wei H, Cui R, Bahr J, Zanesi N, Luo Z, Meng W, et al. miR-130a deregulates PTEN and stimulates tumor growth. Cancer Res. 2017; 77:6168–6178. PMID: 28935812.
Article27. Holen I, Speirs V, Morrissey B, Blyth K. In vivo models in breast cancer research: progress, challenges and future directions. Dis Model Mech. 2017; 10:359–371. PMID: 28381598.28. Rashid OM, Takabe K. Animal models for exploring the pharmacokinetics of breast cancer therapies. Expert Opin Drug Metab Toxicol. 2015; 11:221–230. PMID: 25416501.
Article29. Martins D, Schmitt F. Microenvironment in breast tumorigenesis: friend or foe? Histol Histopathol. 2019; 34:13–24. PMID: 29978449.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Non-Coding RNA NORAD Inhibits Breast Cancer Cell Proliferation and Metastasis by Regulating miR-155-5p/ SOCS1 Axis
- MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN
- MicroRNA-98-5p Inhibits Tumorigenesis of Hepatitis B Virus-Related Hepatocellular Carcinoma by Targeting NF-κB-Inducing Kinase
- Long Noncoding-RNA Component of Mitochondrial RNA Processing Endoribonuclease Promotes Carcinogenesis in Triple-Negative Breast Cancer Cells via the Competing Endogenous RNA Mechanism
- Prolactin Inhibits BCL6 Expression in Breast Cancer Cells through a MicroRNA-339-5p-Dependent Pathway