J Breast Cancer.
2016 Mar;19(1):26-33. 10.4048/jbc.2016.19.1.26.
Prolactin Inhibits BCL6 Expression in Breast Cancer Cells through a MicroRNA-339-5p-Dependent Pathway
- Affiliations
-
- 1Department of Pathology, The Second Affiliated Hospital of Anhui Medical University, Hefei, China. aydjohn@yahoo.com
- 2Department of Pathology, The Second People's Hospital of Hefei, Hefei, China.
- 3Department of Pathology, Anhui Medical University, Hefei, China.
- 4Department of General Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, China.
- KMID: 2176318
- DOI: http://doi.org/10.4048/jbc.2016.19.1.26
Abstract
- PURPOSE
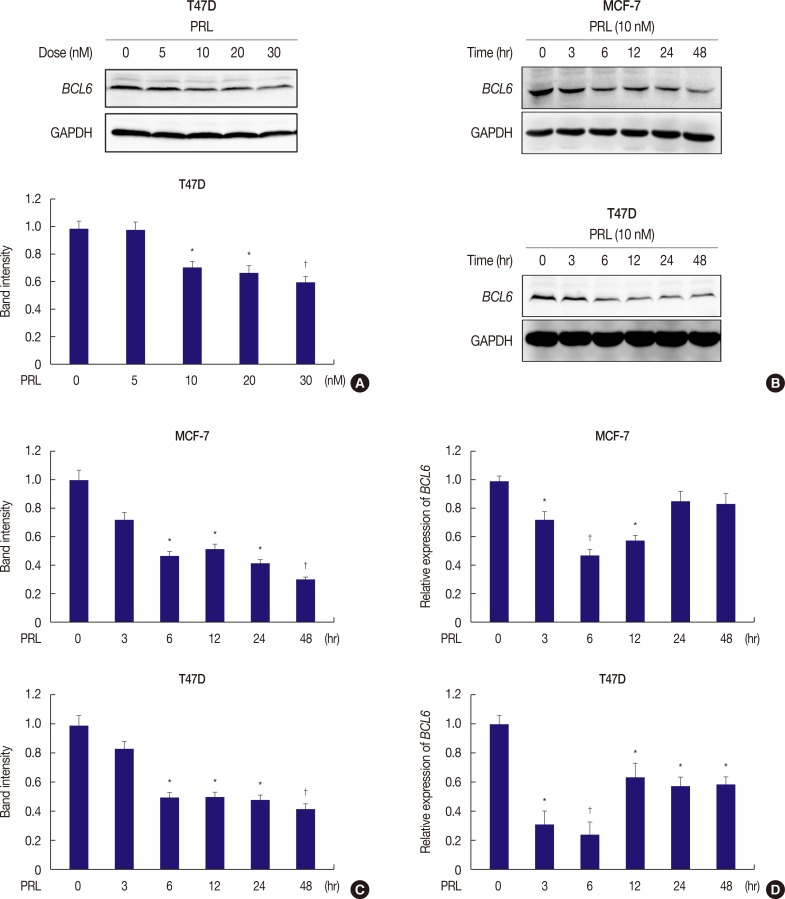
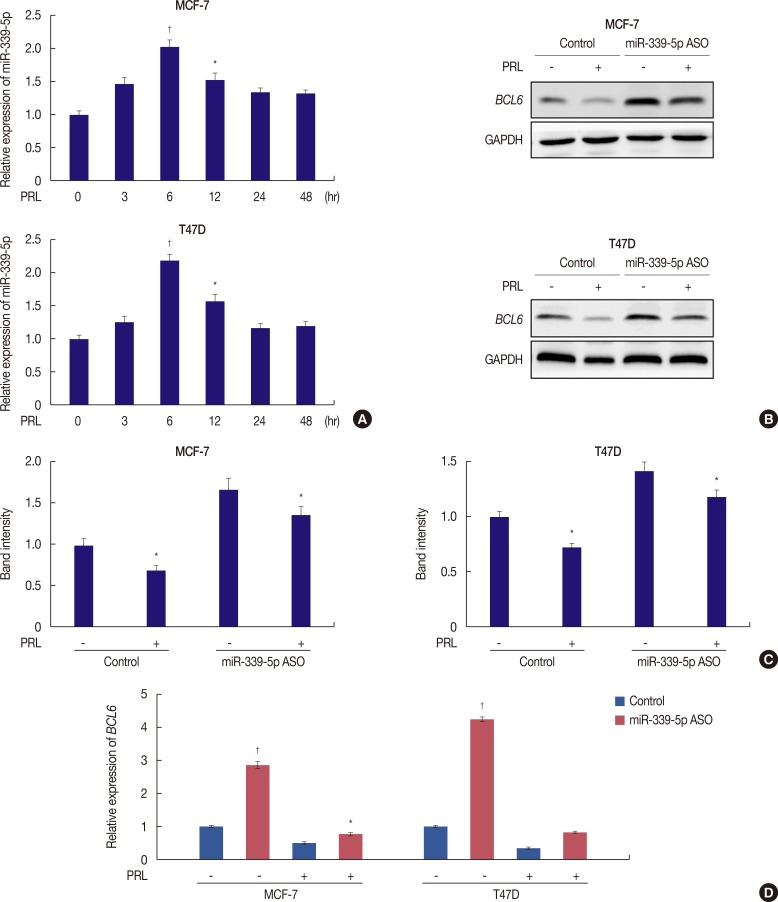
Prolactin (PRL) plays a critical role in breast cancer progression by activating its cognate receptor and promotes the growth and differentiation of breast cancer cells. Studies have shown that B-cell lymphoma 6 (BCL6) is the target gene of microRNA-339-5p (miR-339-5p) and that BCL6 expression contributes to breast cancer progression. Herein, we identified PRL as a potent suppressor of BCL6 expression in human breast cancer cells.
METHODS
Western blotting and quantitative reverse transcription-polymerase chain reaction were used to investigate molecular mechanisms underlying miR-339-5p expression and BCL6 manipulation in MCF-7, T47D, and SKBR3 breast cancer cells. Phenotypic changes in these breast cancer cell lines were assessed by performing cell viability (MTT), colony formation, migration, and invasion assays.
RESULTS
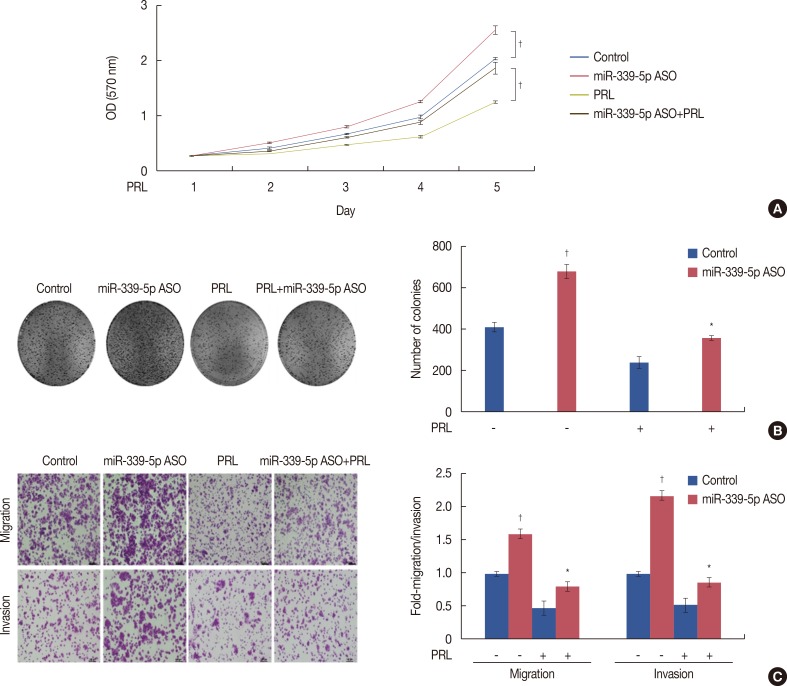
PRL suppressed BCL6 protein and mRNA expression and upregulated miR-339-5p expression in MCF-7 and T47D breast cancer cells. Selective downregulation of miR-339-5p expression significantly reversed PRL-induced suppression of BCL6 mRNA and protein expression. Exogenous PRL stimulation significantly decreased the proliferation, colony formation, migration, and invasion of breast cancer cells, and suppression of miR-339-5p expression reversed these processes in vitro.
CONCLUSION
These results indicated that PRL inhibited BCL6 expression and regulated breast cancer progression through a miR-339-5p-dependent pathway.
MeSH Terms
Figure
Reference
-
1. Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000; 13:199–212. PMID: 10981963.
Article2. Wu Q, Liu X, Yan H, He YH, Ye S, Cheng XW, et al. B-cell lymphoma 6 protein stimulates oncogenicity of human breast cancer cells. BMC Cancer. 2014; 14:418. PMID: 24917186.
Article3. Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009; 136:642–655. PMID: 19239886.
Article4. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–297. PMID: 14744438.5. Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005; 132:4653–4662. PMID: 16224045.
Article6. Gregory RI, Shiekhattar R. MicroRNA biogenesis and cancer. Cancer Res. 2005; 65:3509–3512. PMID: 15867338.7. Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005; 122:6–7. PMID: 16009126.
Article8. Mendell JT. MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell Cycle. 2005; 4:1179–1184. PMID: 16096373.9. Wu ZS, Wu Q, Wang CQ, Wang XN, Wang Y, Zhao JJ, et al. MiR-339-5p inhibits breast cancer cell migration and invasion in vitro and may be a potential biomarker for breast cancer prognosis. BMC Cancer. 2010; 10:542. PMID: 20932331.
Article10. Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003; 24:1–27. PMID: 12588805.
Article11. Tran TH, Utama FE, Lin J, Yang N, Sjolund AB, Ryder A, et al. Prolactin inhibits BCL6 expression in breast cancer through a Stat5a-dependent mechanism. Cancer Res. 2010; 70:1711–1721. PMID: 20124477.
Article12. Wu ZS, Wu Q, Wang CQ, Wang XN, Huang J, Zhao JJ, et al. miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 2011; 117:2842–2852. PMID: 21692045.
Article13. Banerjee A, Wu ZS, Qian P, Kang J, Pandey V, Liu DX, et al. ARTEMIN synergizes with TWIST1 to promote metastasis and poor survival outcome in patients with ER negative mammary carcinoma. Breast Cancer Res. 2011; 13:R112. PMID: 22060274.
Article14. Hennighausen L, Robinson GW, Wagner KU, Liu W. Prolactin signaling in mammary gland development. J Biol Chem. 1997; 272:7567–7569. PMID: 9119818.
Article15. Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA. Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene. 2003; 22:4664–4674. PMID: 12879011.16. Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998; 19:225–268. PMID: 9626554.
Article17. Wagner KU, Krempler A, Triplett AA, Qi Y, George NM, Zhu J, et al. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol. 2004; 24:5510–5520. PMID: 15169911.
Article18. Peck AR, Witkiewicz AK, Liu C, Stringer GA, Klimowicz AC, Pequignot E, et al. Loss of nuclear localized and tyrosine phosphorylated Stat5 in breast cancer predicts poor clinical outcome and increased risk of antiestrogen therapy failure. J Clin Oncol. 2011; 29:2448–2458. PMID: 21576635.
Article19. Takeda N, Arima M, Tsuruoka N, Okada S, Hatano M, Sakamoto A, et al. Bcl6 is a transcriptional repressor for the IL-18 gene. J Immunol. 2003; 171:426–431. PMID: 12817026.20. Fernández de Mattos S, Essafi A, Soeiro I, Pietersen AM, Birkenkamp KU, Edwards CS, et al. FoxO3a and BCR-ABL regulate cyclin D2 transcription through a STAT5/BCL6-dependent mechanism. Mol Cell Biol. 2004; 24:10058–10071. PMID: 15509806.21. Meyer RD, Laz EV, Su T, Waxman DJ. Male-specific hepatic Bcl6: growth hormone-induced block of transcription elongation in females and binding to target genes inversely coordinated with STAT5. Mol Endocrinol. 2009; 23:1914–1926. PMID: 19797429.
Article22. Sato T, Tran TH, Peck AR, Girondo MA, Liu C, Goodman CR, et al. Prolactin suppresses a progestin-induced CK5-positive cell population in luminal breast cancer through inhibition of progestin-driven BCL6 expression. Oncogene. 2014; 33:2215–2224. PMID: 23708665.
Article23. Tworoger SS, Eliassen AH, Zhang X, Qian J, Sluss PM, Rosner BA, et al. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013; 73:4810–4819. PMID: 23783576.
Article24. Tworoger SS, Hankinson SE. Prolactin and breast cancer etiology: an epidemiologic perspective. J Mammary Gland Biol Neoplasia. 2008; 13:41–53. PMID: 18246319.
Article25. Sato T, Tran TH, Peck AR, Liu C, Ertel A, Lin J, et al. Global profiling of prolactin-modulated transcripts in breast cancer in vivo. Mol Cancer. 2013; 12:59. PMID: 23758962.
Article26. Chen Y, Huang K, Chen KE, Walker AM. Prolactin and estradiol utilize distinct mechanisms to increase serine-118 phosphorylation and decrease levels of estrogen receptor alpha in T47D breast cancer cells. Breast Cancer Res Treat. 2010; 120:369–377. PMID: 19377875.27. Rasmussen LM, Frederiksen KS, Din N, Galsgaard E, Christensen L, Berchtold MW, et al. Prolactin and oestrogen synergistically regulate gene expression and proliferation of breast cancer cells. Endocr Relat Cancer. 2010; 17:809–822. PMID: 20601496.
Article28. Zhou C, Liu G, Wang L, Lu Y, Yuan L, Zheng L, et al. MiR-339-5p regulates the growth, colony formation and metastasis of colorectal cancer cells by targeting PRL-1. PLoS One. 2013; 8:e63142. PMID: 23696794.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Non-Coding RNA NORAD Inhibits Breast Cancer Cell Proliferation and Metastasis by Regulating miR-155-5p/ SOCS1 Axis
- MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN
- MicroRNA-98-5p Inhibits Tumorigenesis of Hepatitis B Virus-Related Hepatocellular Carcinoma by Targeting NF-κB-Inducing Kinase
- Perioperative stress prolong post-surgical pain via miR-339-5p targeting oprm1 in the amygdala
- HOXD Antisense Growth-Associated Long Noncoding RNA Promotes TripleNegative Breast Cancer Progression by Activating Wnt Signaling Pathway