Korean J Physiol Pharmacol.
2018 Nov;22(6):697-703. 10.4196/kjpp.2018.22.6.697.
Mitochondrial dysfunction reduces the activity of KIR2.1 K⺠channel in myoblasts via impaired oxidative phosphorylation
- Affiliations
-
- 1Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 03080, Korea.
- 2Department of Physiology, Dongguk University College of Medicine, Gyeongju 38066, Korea. jhnam@dongguk.ac.kr
- 3Channelopathy Research Center (CRC), Dongguk University College of Medicine, Goyang 10326, Korea. wanlee@dongguk.ac.kr
- 4Department of Medical Biotechnology, Yeungnam University, Gyeongsan 38541, Korea.
- 5Department of Biochemistry, Dongguk University College of Medicine, Gyeongju 38066, Korea.
- KMID: 2430092
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.697
Abstract
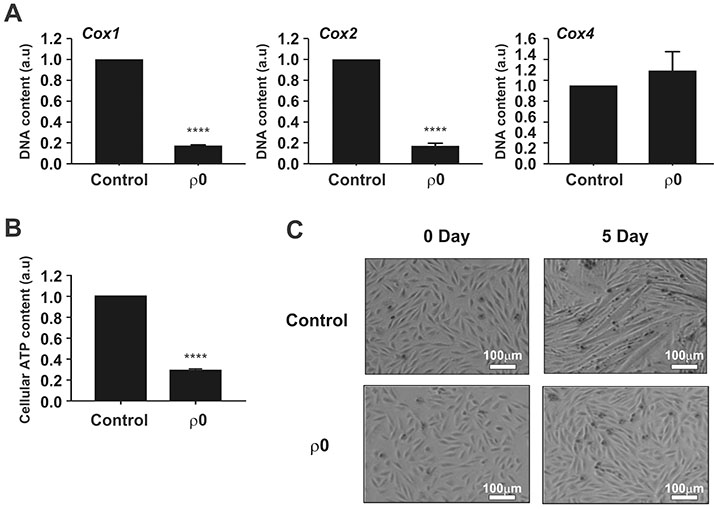
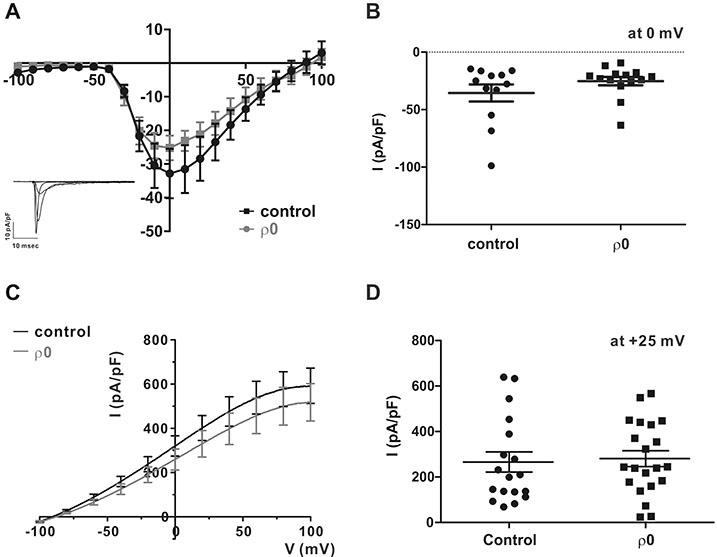
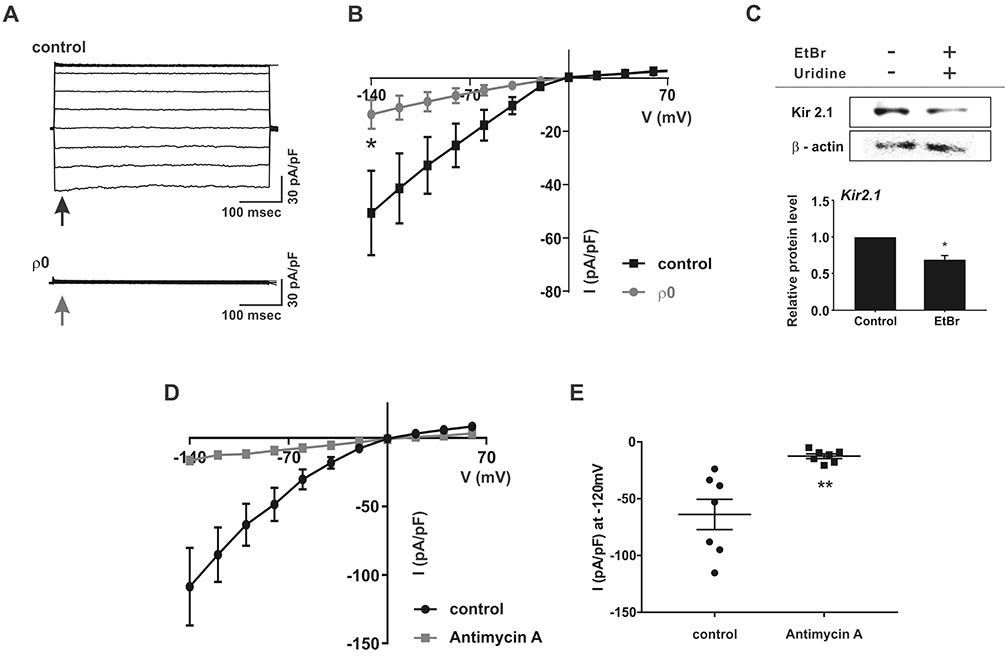
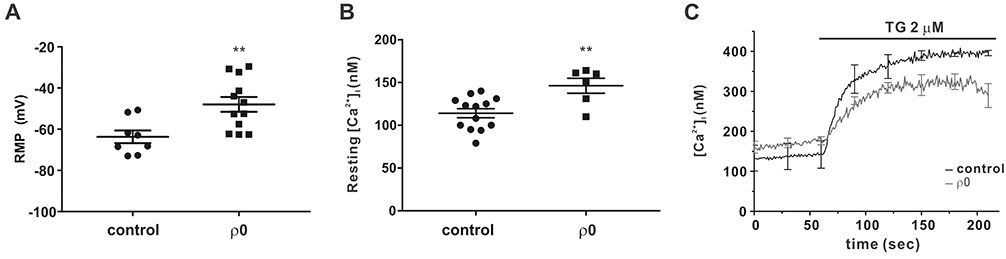
- Myoblast fusion depends on mitochondrial integrity and intracellular Ca²âº signaling regulated by various ion channels. In this study, we investigated the ionic currents associated with [Ca²âº]i regulation in normal and mitochondrial DNA-depleted (Ï0) L6 myoblasts. The Ï0 myoblasts showed impaired myotube formation. The inwardly rectifying K⺠current (I(Kir)) was largely decreased with reduced expression of KIR2.1, whereas the voltage-operated Ca²âº channel and Ca²âº-activated K⺠channel currents were intact. Sustained inhibition of mitochondrial electron transport by antimycin A treatment (24 h) also decreased the I(Kir). The Ï0 myoblasts showed depolarized resting membrane potential and higher basal [Ca²âº]áµ¢. Our results demonstrated the specific downregulation of I(Kir) by dysfunctional mitochondria. The resultant depolarization and altered Ca²âº signaling might be associated with impaired myoblast fusion in Ï0 myoblasts.
Keyword
MeSH Terms
Figure
Reference
-
1. Abmayr SM, Pavlath GK. Myoblast fusion: lessons from flies and mice. Development. 2012; 139:641–656.
Article2. Buckingham M. Skeletal muscle formation in vertebrates. Curr Opin Genet Dev. 2001; 11:440–448.
Article3. David JD, See WM, Higginbotham CA. Fusion of chick embryo skeletal myoblasts: role of calcium influx preceding membrane union. Dev Biol. 1981; 82:297–307.
Article4. Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998; 14:167–196.
Article5. Friday BB, Pavlath GK. A calcineurin- and NFAT-dependent pathway regulates Myf5 gene expression in skeletal muscle reserve cells. J Cell Sci. 2001; 114:303–310.
Article6. Molkentin JD, Olson EN. Combinatorial control of muscle development by basic helix-loop-helix and MADS-box transcription factors. Proc Natl Acad Sci U S A. 1996; 93:9366–9373.
Article7. Darbellay B, Arnaudeau S, König S, Jousset H, Bader C, Demaurex N, Bernheim L. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem. 2009; 284:5370–5380.
Article8. Cooper E. A new role for ion channels in myoblast fusion. J Cell Biol. 2001; 153:F9–F12.
Article9. Bijlenga P, Liu JH, Espinos E, Haenggeli CA, Fischer-Lougheed J, Bader CR, Bernheim L. T-type alpha 1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc Natl Acad Sci U S A. 2000; 97:7627–7632.10. Fischer-Lougheed J, Liu JH, Espinos E, Mordasini D, Bader CR, Belin D, Bernheim L. Human myoblast fusion requires expression of functional inward rectifier Kir2.1 channels. J Cell Biol. 2001; 153:677–686.
Article11. Konig S, Béguet A, Bader CR, Bernheim L. The calcineurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development. 2006; 133:3107–3114.12. Tanaka S, Ono Y, Sakamoto K. DCEBIO facilitates myogenic differentiation via intermediate conductance Ca2+ activated K+ channel activation in C2C12 myoblasts. J Pharmacol Sci. 2017; 133:276–279.13. Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014; 505:335–343.
Article14. Wagatsuma A, Sakuma K. Mitochondria as a potential regulator of myogenesis. ScientificWorldJournal. 2013; 2013:593267.
Article15. Biswas G, Adebanjo OA, Freedman BD, Anandatheerthavarada HK, Vijayasarathy C, Zaidi M, Kotlikoff M, Avadhani NG. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of interorganelle crosstalk. EMBO J. 1999; 18:522–533.16. Park SY, Choi GH, Choi HI, Ryu J, Jung CY, Lee W. Depletion of mitochondrial DNA causes impaired glucose utilization and insulin resistance in L6 GLUT4myc myocytes. J Biol Chem. 2005; 280:9855–9864.
Article17. Nam JH, Lee DU. Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J Dermatol Sci. 2016; 84:305–313.
Article18. Ma X, Jin M, Cai Y, Xia H, Long K, Liu J, Yu Q, Yuan J. Mitochondrial electron transport chain complex III is required for antimycin A to inhibit autophagy. Chem Biol. 2011; 18:1474–1481.
Article19. Okada Y. Patch clamp techniques: from beginning to advanced protocols. Tokyo: Springer;2012.20. Bernheim L, Bader CR. Human myoblast differentiation: Ca2+ channels are activated by K+ channels. News Physiol Sci. 2002; 17:22–26.21. D'Avanzo N, Cheng WW, Doyle DA, Nichols CG. Direct and specific activation of human inward rectifier K+ channels by membrane phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2010; 285:37129–37132.22. Kim KS, Jang JH, Lin H, Choi SW, Kim HR, Shin DH, Nam JH, Zhang YH, Kim SJ. Rise and fall of Kir2.2 current by TLR4 signaling in human monocytes: PKC-dependent trafficking and PI3K-mediated PIP2 decrease. J Immunol. 2015; 195:3345–3354.23. Alesutan I, Munoz C, Sopjani M, Dërmaku-Sopjani M, Michael D, Fraser S, Kemp BE, Seebohm G, Föller M, Lang F. Inhibition of Kir2.1 (KCNJ2) by the AMP-activated protein kinase. Biochem Biophys Res Commun. 2011; 408:505–510.
Article24. Zhao B, Qiang L, Joseph J, Kalyanaraman B, Viollet B, He YY. Mitochondrial dysfunction activates the AMPK signaling and autophagy to promote cell survival. Genes Dis. 2016; 3:82–87.
Article25. Engbers JD, Anderson D, Zamponi GW, Turner RW. Signal processing by T-type calcium channel interactions in the cerebellum. Front Cell Neurosci. 2013; 7:230.
Article26. Lee N, Jeong S, Kim KC, Kim JA, Park JY, Kang HW, Perez-Reyes E, Lee JH. Ca2+ egulation of Cav3.3 T-type Ca2+ channel is mediated by calmodulin. Mol Pharmacol. 2017; 92:347–357.27. Scrimgeour NR, Wilson DP, Barritt GJ, Rychkov GY. Structural and stoichiometric determinants of Ca2+ release-activated Ca2+ (CRAC) channel Ca2+-dependent inactivation. Biochim Biophys Acta. 2014; 1838:1281–1287.