Int J Stem Cells.
2018 Nov;11(2):227-234. 10.15283/ijsc18053.
The Pharmacological Inhibition of ERK5 Enhances Apoptosis in Acute Myeloid Leukemia Cells
- Affiliations
-
- 1Department of Stem Cell Biology, School of Medicine, Konkuk University, Seoul, Korea. stemchung@gmail.com
- 2Stem Cell Research Center, Jeju National University, Jeju, Korea.
- 3Mirae Cell Bio Co. LTD, Seoul, Korea.
- KMID: 2429555
- DOI: http://doi.org/10.15283/ijsc18053
Abstract
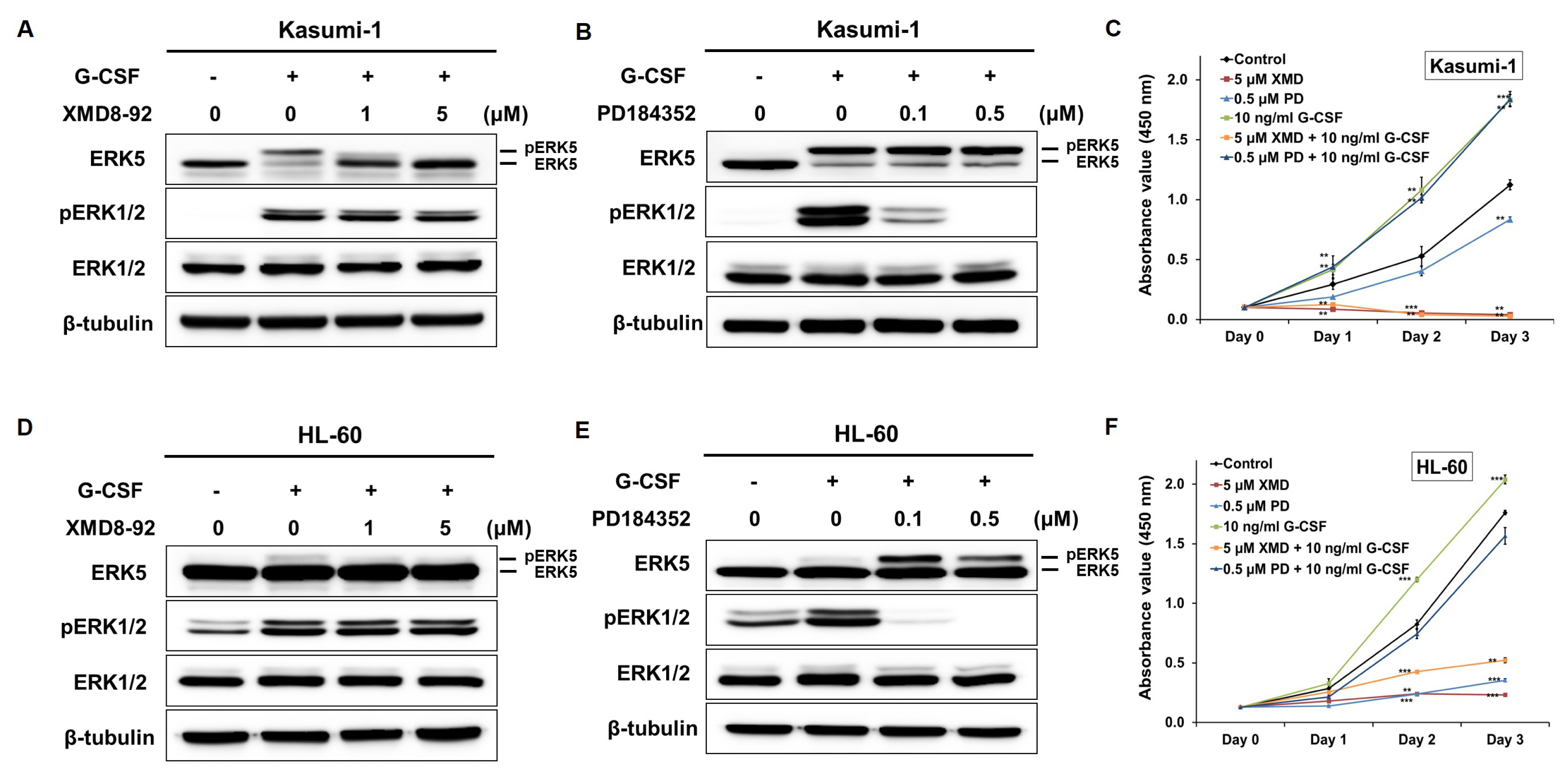
- Acute myeloid leukemia (AML) is a fatal hematological malignancy which is resistant to a variety of chemotherapy drugs. Extracellular signal-regulated kinase 5 (ERK5) plays a novel role in chemoresistance in some cancer cells and this pathway is a central mediator of cell survival and apoptotic regulation. The aim of this study was to investigate the effect of ERK5 inhibitor, XMD8-92, on proliferation and apoptosis in AML cell lines. Findings showed that XMD8-92 inhibited the activation of ERK5 by G-CSF and decreased the expression of c-Myc and Cyclin D1. The treatment of XMD8-92 reduced the phosphorylation of ERK5 leading to a distinct inhibition of cell proliferation and increased apoptosis in Kasumi-1 and HL-60 cells. Taken together, our study suggests that the inhibition of ERK5 by XMD8-92 can trigger apoptosis and inhibit proliferation in AMLs. Therefore, the inhibition of ERK5 may be an effective adjuvant in AML chemotherapy.
Keyword
MeSH Terms
-
Apoptosis*
Cell Cycle
Cell Line
Cell Proliferation
Cell Survival
Cyclin D1
Drug Therapy
Granulocyte Colony-Stimulating Factor
Hematologic Neoplasms
HL-60 Cells
Humans
Leukemia, Myeloid, Acute*
Mitogen-Activated Protein Kinase 7
Phosphorylation
Cyclin D1
Granulocyte Colony-Stimulating Factor
Mitogen-Activated Protein Kinase 7
Figure
Reference
-
References
1. Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007; 25:1908–1915. DOI: 10.1200/JCO.2006.10.2731. PMID: 17488990.
Article2. Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, Tidefelt U, Wahlin A, Hoglund M. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish acute leukemia registry. Blood. 2009; 113:4179–4187. DOI: 10.1182/blood-2008-07-172007.
Article3. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001; 410:37–40. DOI: 10.1038/35065000. PMID: 11242034.
Article4. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002; 298:1911–1912. DOI: 10.1126/science.1072682. PMID: 12471242.
Article5. Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007; 26:3100–3112. DOI: 10.1038/sj.onc.1210392. PMID: 17496909.
Article6. Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. Embo J. 1997; 16:7054–7066. DOI: 10.1093/emboj/16.23.7054.
Article7. Morimoto H, Kondoh K, Nishimoto S, Terasawa K, Nishida E. Activation of a C-terminal transcriptional activation domain of ERK5 by autophosphorylation. J Biol Chem. 2007; 282:35449–35456. DOI: 10.1074/jbc.M704079200. PMID: 17928297.
Article8. Yan C, Luo H, Lee JD, Abe J, Berk BC. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J Biol Chem. 2001; 276:10870–10878. DOI: 10.1074/jbc.M009286200. PMID: 11139578.
Article9. Deng X, Yang Q, Kwiatkowski N, Sim T, McDermott U, Settleman JE, Lee JD, Gray NS. Discovery of a benzo[ e]pyrimido-[5,4-b][1,4]diazepin-6(11H)-one as a potent and selective inhibitor of big MAP kinase 1. ACS Med Chem Lett. 2011; 2:195–200. DOI: 10.1021/ml100304b. PMID: 21412406. PMCID: 3055678.
Article10. Yang Q, Deng X, Lu B, Cameron M, Fearns C, Patricelli MP, Yates JR 3rd, Gray NS, Lee JD. Pharmacological inhibition of BMK1 suppresses tumor growth through promyelocytic leukemia protein. Cancer Cell. 2010; 18:258–267. DOI: 10.1016/j.ccr.2010.08.008. PMID: 20832753. PMCID: 2939729.
Article11. Williams CA, Fernandez-Alonso R, Wang J, Toth R, Gray NS, Findlay GM. Erk5 Is a key regulator of naive-primed transition and embryonic stem cell identity. Cell Rep. 2016; 16:1820–1828. DOI: 10.1016/j.celrep.2016.07.033. PMID: 27498864. PMCID: 4987282.
Article12. Perez-Madrigal D, Finegan KG, Paramo B, Tournier C. The extracellular-regulated protein kinase 5 (ERK5) promotes cell proliferation through the down-regulation of inhibitors of cyclin dependent protein kinases (CDKs). Cell Signal. 2012; 24:2360–2368. DOI: 10.1016/j.cellsig.2012.08.001. PMID: 22917534.
Article13. Carvajal-Vergara X, Tabera S, Montero JC, Esparis-Ogando A, Lopez-Perez R, Mateo G, Gutierrez N, Parmo-Cabanas M, Teixido J, San Miguel JF, Pandiella A. Multifunctional role of Erk5 in multiple myeloma. Blood. 2005; 105:4492–4499. DOI: 10.1182/blood-2004-08-2985. PMID: 15692064.
Article14. Nagel S, Burek C, Venturini L, Scherr M, Quentmeier H, Meyer C, Rosenwald A, Drexler HG, MacLeod RA. Comprehensive analysis of homeobox genes in Hodgkin lymphoma cell lines identifies dysregulated expression of HOXB9 mediated via ERK5 signaling and BMI1. Blood. 2007; 109:3015–3023.
Article15. Razumovskaya E, Sun J, Ronnstrand L. Inhibition of MEK5 by BIX02188 induces apoptosis in cells expressing the oncogenic mutant FLT3-ITD. Biochem Biophys Res Commun. 2011; 412:307–312. DOI: 10.1016/j.bbrc.2011.07.089. PMID: 21820407.
Article16. Zheng R, Studzinski GP. Optimal AraC-Cytotoxicity to AML Cells requires ERK5 activity. J Cell Biochem. 2017; 118:1583–1589. DOI: 10.1002/jcb.25820.
Article17. Xiong Y, Zhang L, Wang T. Phosphorylation of BMK1 induces prostatic carcinoma cell proliferation by promoting entry into the S phase of the cell cycle. Oncol Lett. 2016; 11:99–104. DOI: 10.3892/ol.2015.3909. PMID: 26870175. PMCID: 4727042.
Article18. Obara Y, Nakahata N. The signaling pathway leading to extracellular signal-regulated kinase 5 (ERK5) activation via G-proteins and ERK5-dependent neurotrophic effects. Mol Pharmacol. 2010; 77:10–16. DOI: 10.1124/mol.109.060236.
Article19. Dong F, Gutkind JS, Larner AC. Granulocyte colony-stimulating factor induces ERK5 activation, which is differentially regulated by protein-tyrosine kinases and protein kinase C. Regulation of cell proliferation and survival. J Biol Chem. 2001; 276:10811–10816. DOI: 10.1074/jbc.M008748200. PMID: 11278431.
Article20. Yan C, Takahashi M, Okuda M, Lee JD, Berk BC. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells. Dependence on tyrosine kinases and intracellular calcium. J Biol Chem. 1999; 274:143–150. DOI: 10.1074/jbc.274.1.143.
Article21. Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998; 93:841–850. DOI: 10.1016/S0092-8674(00)81444-0. PMID: 9630227.
Article22. Dong F, Liu X, de Koning JP, Touw IP, Hennighausen L, Larner A, Grimley PM. Stimulation of Stat5 by granulocyte colony-stimulating factor (G-CSF) is modulated by two distinct cytoplasmic regions of the G-CSF receptor. J Immunol. 1998; 161:6503–6509. PMID: 9862674.23. Moschos SJ, Sullivan RJ, Hwu WJ, Ramanathan RK, Adjei AA, Fong PC, Shapira-Frommer R, Tawbi HA, Rubino J, Rush TS 3rd, Zhang D, Miselis NR, Samatar AA, Chun P, Rubin EH, Schiller J, Long BJ, Dayananth P, Carr D, Kirschmeier P, Bishop WR, Deng Y, Cooper A, Shipps GW, Moreno BH, Robert L, Ribas A, Flaherty KT. Development of MK-8353, an orally administered ERK1/2 inhibitor, in patients with advanced solid tumors. JCI Insight. 2018; DOI: 10.1172/jci.insight.92352. [Epub ahead of print]. PMID: 29467321. PMCID: 5916243.
Article24. Sureban SM, May R, Weygant N, Qu D, Chandrakesan P, Bannerman-Menson E, Ali N, Pantazis P, Westphalen CB, Wang TC, Houchen CW. XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 2014; 351:151–161. DOI: 10.1016/j.canlet.2014.05.011. PMID: 24880079.
Article25. Montero JC, Ocana A, Abad M, Ortiz-Ruiz MJ, Pandiella A, Esparis-Ogando A. Expression of Erk5 in early stage breast cancer and association with disease free survival identifies this kinase as a potential therapeutic target. PLoS One. 2009; 4:e5565. DOI: 10.1371/journal.pone.0005565. PMID: 19440538. PMCID: 2678256.
Article26. Villa-Moruzzi E. Tyrosine phosphatases in the HER2-directed motility of ovarian cancer cells: Involvement of PTPN12, ERK5 and FAK. Anal Cell Pathol (Amst). 2011; 34:101–112. DOI: 10.1155/2011/870459.
Article27. McCracken SR, Ramsay A, Heer R, Mathers ME, Jenkins BL, Edwards J, Robson CN, Marquez R, Cohen P, Leung HY. Aberrant expression of extracellular signal-regulated kinase 5 in human prostate cancer. Oncogene. 2008; 27:2978–2988. DOI: 10.1038/sj.onc.1210963.
Article28. Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999; 274:26563–26571. DOI: 10.1074/jbc.274.37.26563. PMID: 10473620.29. Terasawa K, Okazaki K, Nishida E. Regulation of c-Fos and Fra-1 by the MEK5-ERK5 pathway. Genes Cells. 2003; 8:263–273. DOI: 10.1046/j.1365-2443.2003.00631.x. PMID: 12622723.
Article30. Mulloy R, Salinas S, Philips A, Hipskind RA. Activation of cyclin D1 expression by the ERK5 cascade. Oncogene. 2003; 22:5387–5398. DOI: 10.1038/sj.onc.1206839. PMID: 12934098.
Article31. Hayashi M, Tapping RI, Chao TH, Lo JF, King CC, Yang Y, Lee JD. BMK1 mediates growth factor-induced cell proliferation through direct cellular activation of serum and glucocorticoid-inducible kinase. J Biol Chem. 2001; 276:8631–8634. DOI: 10.1074/jbc.C000838200. PMID: 11254654.
Article32. Ranganathan A, Pearson GW, Chrestensen CA, Sturgill TW, Cobb MH. The MAP kinase ERK5 binds to and phosphorylates p90 RSK. Arch Biochem Biophys. 2006; 449:8–16. DOI: 10.1016/j.abb.2006.02.023. PMID: 16626623.
Article33. Garaude J, Cherni S, Kaminski S, Delepine E, Chable- Bessia C, Benkirane M, Borges J, Pandiella A, Iniguez MA, Fresno M, Hipskind RA, Villalba M. ERK5 activates NF-kappaB in leukemic T cells and is essential for their growth in vivo. J Immunol. 2006; 177:7607–7617. DOI: 10.4049/jimmunol.177.11.7607. PMID: 17114430.
Article34. English JM, Pearson G, Baer R, Cobb MH. Identification of substrates and regulators of the mitogen-activated protein kinase ERK5 using chimeric protein kinases. J Biol Chem. 1998; 273:3854–3860. DOI: 10.1074/jbc.273.7.3854. PMID: 9461566.
Article35. Kesavan K, Lobel-Rice K, Sun W, Lapadat R, Webb S, Johnson GL, Garrington TP. MEKK2 regulates the coordinate activation of ERK5 and JNK in response to FGF-2 in fibroblasts. J Cell Physiol. 2004; 199:140–148. DOI: 10.1002/jcp.10457. PMID: 14978743.
Article36. Lennartsson J, Burovic F, Witek B, Jurek A, Heldin CH. Erk 5 is necessary for sustained PDGF-induced Akt phosphorylation and inhibition of apoptosis. Cell Signal. 2010; 22:955–960. DOI: 10.1016/j.cellsig.2010.01.020. PMID: 20138986.
Article37. Del Poeta G, Venditti A, Del Principe MI, Maurillo L, Buccisano F, Tamburini A, Cox MC, Franchi A, Bruno A, Mazzone C, Panetta P, Suppo G, Masi M, Amadori S. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood. 2003; 101:2125–2131. DOI: 10.1182/blood-2002-06-1714.
Article38. Marcucci G, Stock W, Dai G, Klisovic RB, Liu S, Klisovic MI, Blum W, Kefauver C, Sher DA, Green M, Moran M, Maharry K, Novick S, Bloomfield CD, Zwiebel JA, Larson RA, Grever MR, Chan KK, Byrd JC. Phase I study of oblimersen sodium, an antisense to Bcl-2, in untreated older patients with acute myeloid leukemia: pharmacokinetics, pharmacodynamics, and clinical activity. J Clin Oncol. 2005; 23:3404–3411. DOI: 10.1200/JCO.2005.09.118. PMID: 15824414.
Article39. Milella M, Estrov Z, Kornblau SM, Carter BZ, Konopleva M, Tari A, Schober WD, Harris D, Leysath CE, Lopez-Berestein G, Huang Z, Andreeff M. Synergistic induction of apoptosis by simultaneous disruption of the Bcl-2 and MEK/MAPK pathways in acute myelogenous leukemia. Blood. 2002; 99:3461–3464. DOI: 10.1182/blood.V99.9.3461. PMID: 11964319.
Article40. Weldon CB, Scandurro AB, Rolfe KW, Clayton JL, Elliott S, Butler NN, Melnik LI, Alam J, McLachlan JA, Jaffe BM, Beckman BS, Burow ME. Identification of mitogen-activated protein kinase kinase as a chemoresistant pathway in MCF-7 cells by using gene expression microarray. Surgery. 2002; 132:293–301. DOI: 10.1067/msy.2002.125389. PMID: 12219026.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Bromodomain Inhibitor JQ1 Enhances the Responses to All-trans Retinoic Acid in HL-60 and MV4-11 Leukemia Cells
- Aurora A kinase expression is increased in leukemia stem cells, and a selective Aurora A kinase inhibitor enhances Ara-C-induced apoptosis in acute myeloid leukemia stem cells
- Laminar flow activation of ERK5 leads to cytoprotective effect via CHIP-mediated p53 ubiquitination in endothelial cells
- Intraparenchymal Myeloid Sarcoma and Subsequent Spinal Myeloid Sarcoma for Acute Myeloblastic Leukemia
- Acute Myeloid Leukemia with Intracardiac Thrombus Presenting as Acute Limb Ischemia