Int J Stem Cells.
2018 Nov;11(2):205-215. 10.15283/ijsc18002.
Effect of Stem Cells and Gene Transfected Stem Cells Therapy on the Pancreas of Experimentally Induced Type 1 Diabetes
- Affiliations
-
- 1Department of Medical Histology & Cell Biology, Faculty of Medicine, Cairo University, Cairo, Egypt. mahakaah2004@yahoo.com
- 2Faculty of Oral and Dental Medicine, Future University, Cairo, Egypt (FUE).
- 3Clinical Pharmacy, Near East University North Cyprus, German University in Cairo, Cairo, Egypt.
- KMID: 2429553
- DOI: http://doi.org/10.15283/ijsc18002
Abstract
- BACKGROUND AND OBJECTIVES
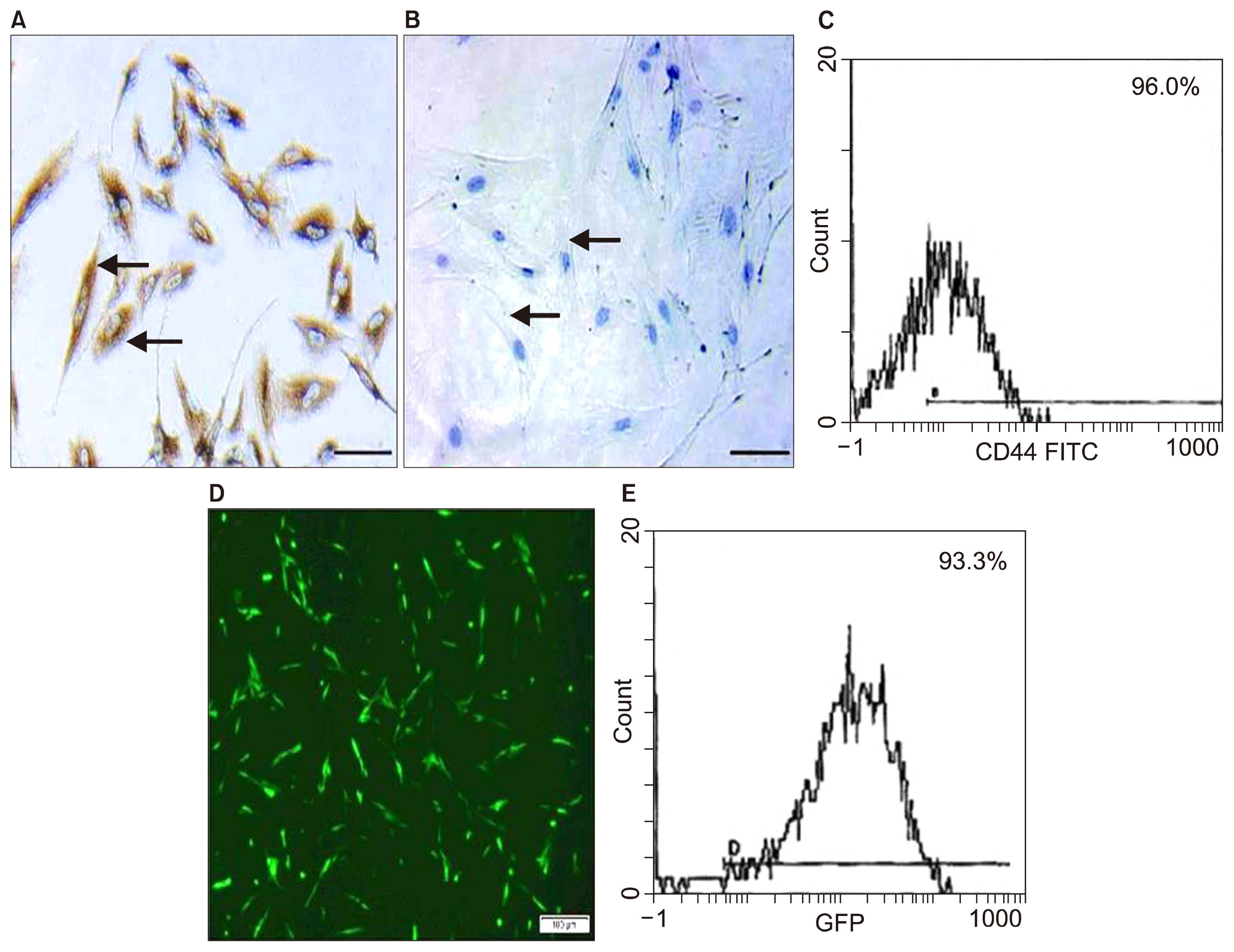
Insulin secretion entirely depends on Ca²âº influx and sequestration into endoplasmic reticulum (ER) of β-cells, performed by Sarco-ER Ca²âº-ATPase 2b (SERCA2b). In diabetes, SERCA2b is decreased in the β-cells leading to impaired intracellular Ca²âº homeostasis and insulin secretion. Adipose mesenchymal stem cells (AMSCs) play a potential role in transplantation in animal models. The present study aimed at investigating and comparing the therapeutic effect of non-transfected AMSCs and SERCA2b gene transfected AMSCs on the pancreas of induced diabetes type 1 in rat.
METHODS AND RESULTS
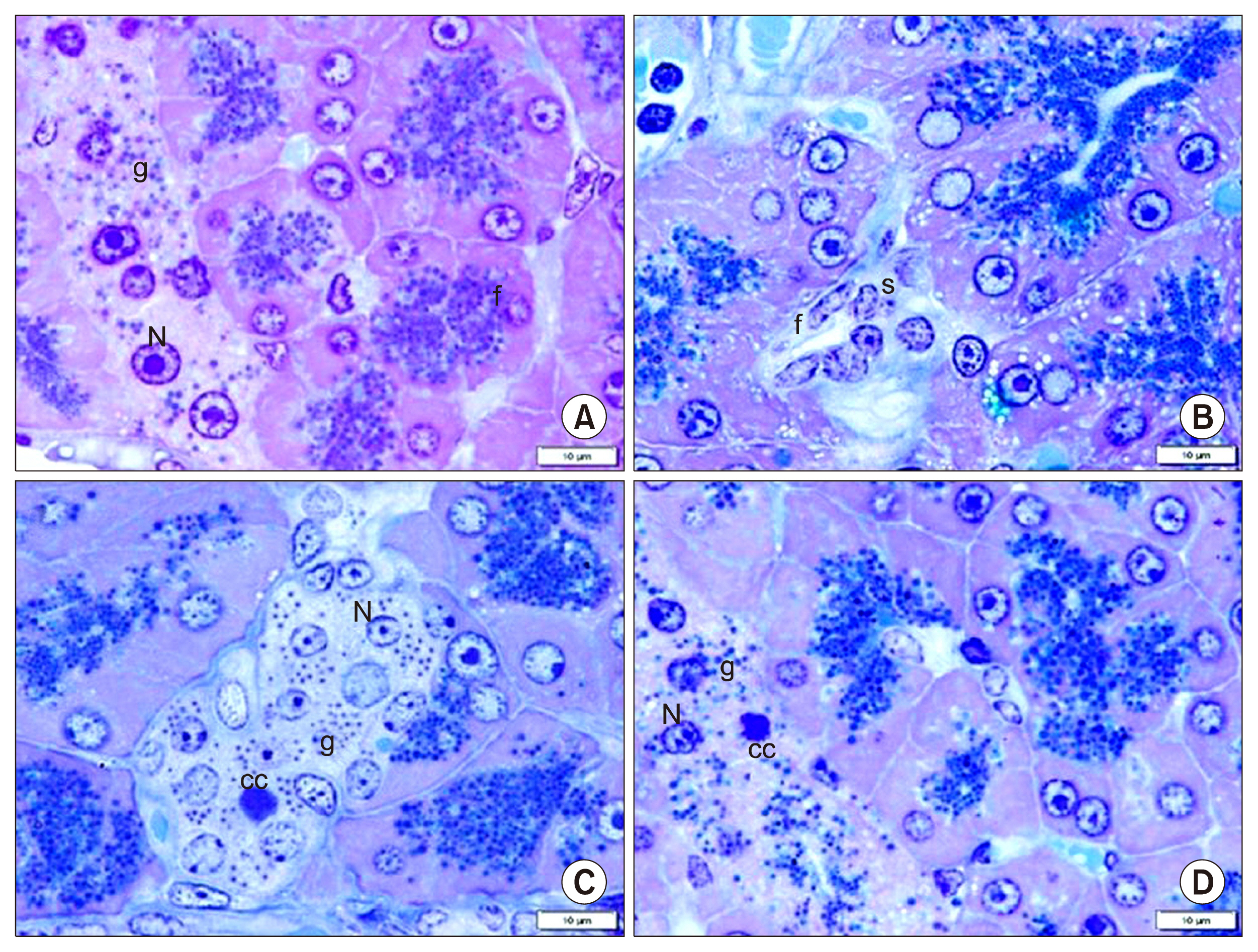
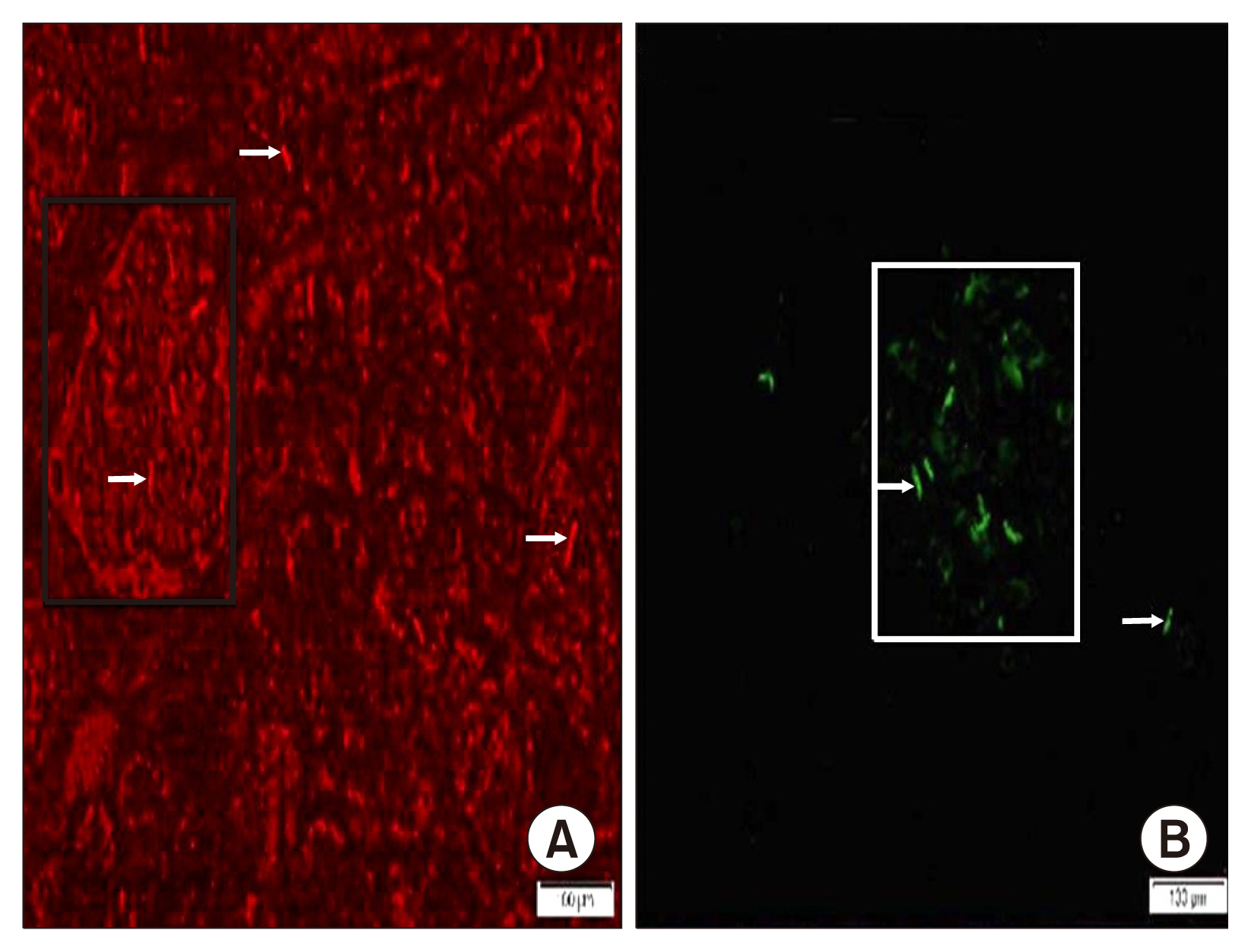
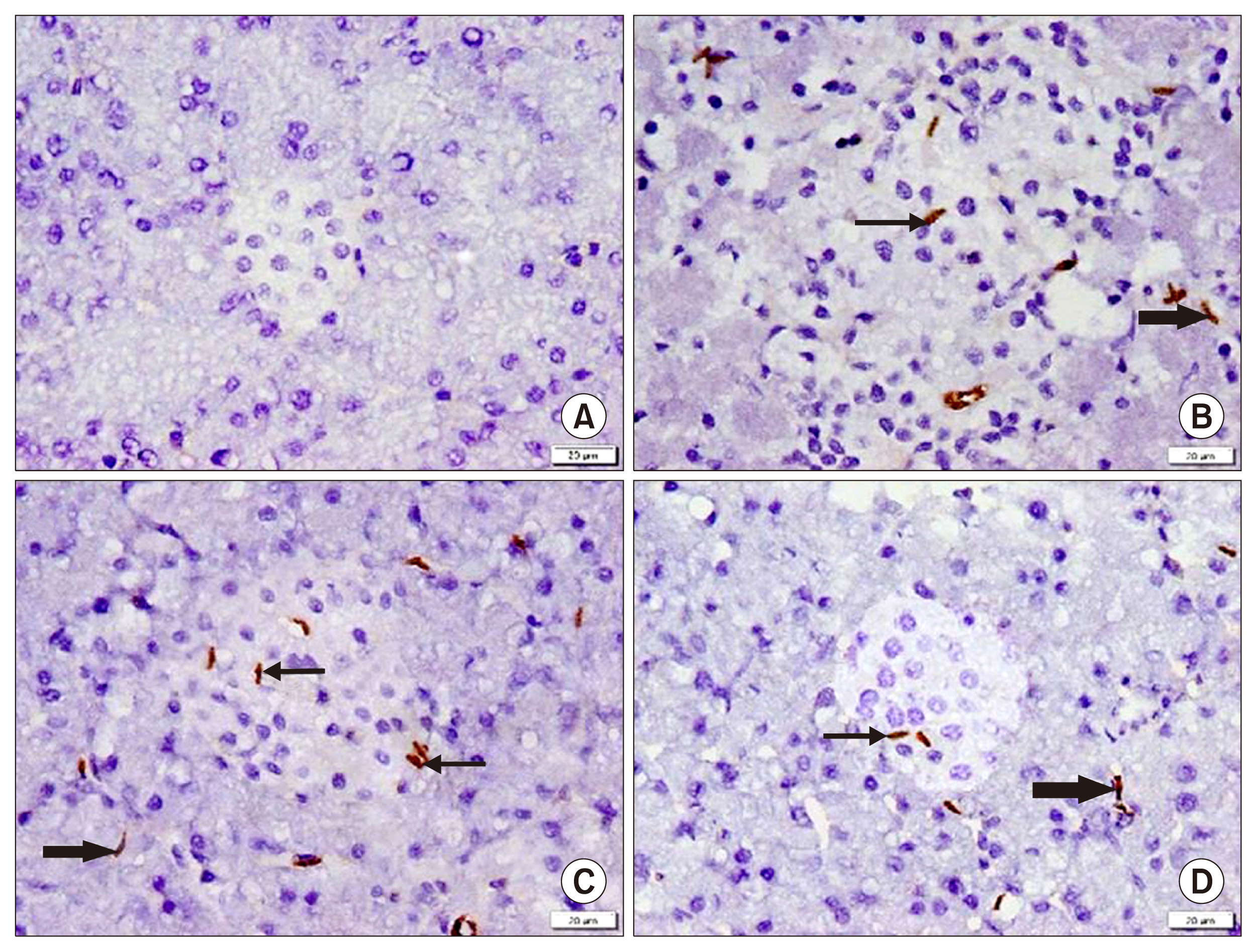
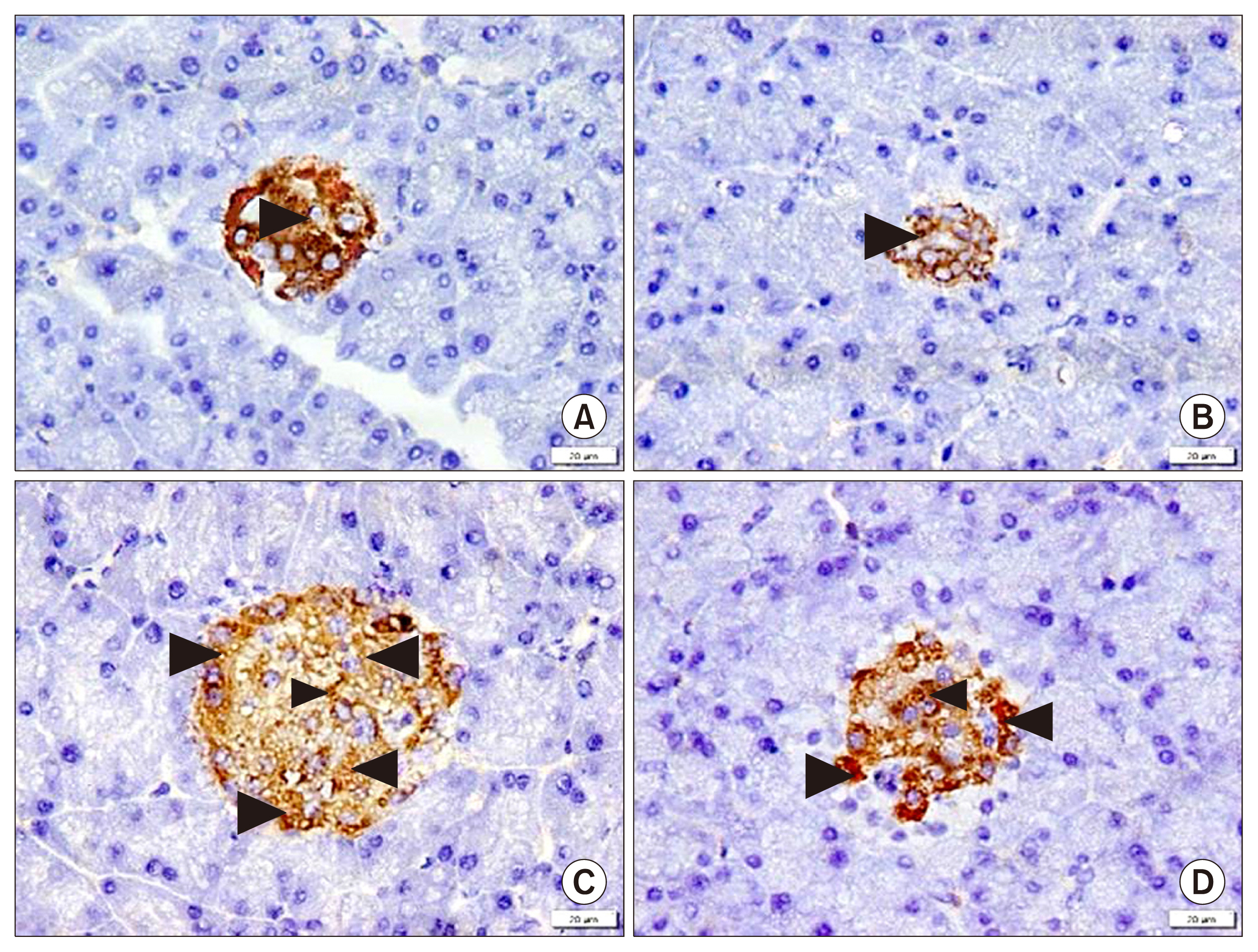
58 adult male albino rats were divided into: Donor group: 22 rats, 2 for isolation, propagation and characterization of AMSCs and SERCA2b transfected AMSCs, in addition 20 for isolated islet calcium level assessment. Group I (Control Group): 6 rats, Group II (Diabetic Group): 10 rats, 50 mg streptozotocin (STZ) were injected intraperitoneal (IP), Group III (AMSCs Group): 10 rats, 1×10ⶠAMSCs were injected intravenous and Group IV (SERCA2b transfected AMSCs Group): 10 rats, 1×10â¶SERCA2b transfected AMSCs were injected as in group III. Groups I, II, III and IV were sacrified 3 weeks following confirmation of diabetes. Serological, histological, morphometric studies and quantitative polymerase chain reaction (qPCR) were performed. Nuclear, cytoplasmic degenerative and extensive fibrotic changes were detected in the islets of group II that regressed in groups III and IV. Isolated islet calcium, blood glucose, plasma insulin and qPCR were confirmative.
CONCLUSIONS
AMSCs and SERCA2b gene transfected AMSCs therapy proved definite therapeutic effect, more obvious in response to SERCA2b gene transfected AMSCs.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011; 94:311–321. DOI: 10.1016/j.diabres.2011.10.029. PMID: 22079683.
Article2. Mohamed SS, Ali EAI, Hosny S. The antidiabetic effect of mesenchymal stem cells vs. nigella sativa oil on streptozotocin induced type 1 diabetic rats. J Cell Sci Ther. 2015; 6:226–235. DOI: 10.4172/2157-7013.1000226.
Article3. Roh SS, Kwon OJ, Yang JH, Kim YS, Lee SH, Jin JS, Jeon YD, Yokozawa T, Kim HJ. Allium hookeri root protects oxidative stress-induced inflammatory responses and β-cell damage in pancreas of streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2016; 16:63. DOI: 10.1186/s12906-016-1032-1.
Article4. Hashemian SJ, Kouhnavard M, Nasli-Esfahani E. Mesenchymal stem cells: rising concerns over their application in treatment of type one diabetes mellitus. J Diabetes Res. 2015; DOI: 10.1155/2015/675103. PMID: 26576437. PMCID: 4630398.
Article5. Zhang W, Schmull S, Du M, Liu J, Lu Z, Zhu H, Xue S, Lian F. Estrogen receptor α and β in mouse: adipose-derived stem cell proliferation, migration, and brown adipogenesis in vitro. Cell Physiol Biochem. 2016; 38:2285–2299. DOI: 10.1159/000445583.
Article6. Zarain-Herzberg A, García-Rivas G, Estrada-Avilés R. Regulation of SERCA pumps expression in diabetes. Cell Calcium. 2014; 56:302–310. DOI: 10.1016/j.ceca.2014.09.005. PMID: 25270119.
Article7. Yu X, Liang X, Xie H, Kumar S, Ravinder N, Potter J, de Mollerat du Jeu X, Chesnut JD. Improved delivery of Cas9 protein/gRNA complexes using lipofectamine CRISPRMAX. Biotechnol Lett. 2016; 38:919–929. DOI: 10.1007/s10529-016-2064-9. PMID: 26892225. PMCID: 4853464.
Article8. Hidaka R, Machida M, Fujimaki S, Terashima K, Asashima M, Kuwabara T. Monitoring neurodegeneration in diabetes using adult neural stem cells derived from the olfactory bulb. Stem Cell Res Ther. 2013; 4:51. DOI: 10.1186/scrt201. PMID: 23673084. PMCID: 3707061.
Article9. Bhansali S, Kumar V, Saikia UN, Medhi B, Jha V, Bhansali A, Dutta P. Effect of mesenchymal stem cells transplantation on glycaemic profile & their localization in streptozotocin induced diabetic Wistar rats. Indian J Med Res. 2015; 142:63–71. DOI: 10.4103/0971-5916.162116. PMID: 26261168. PMCID: 4557252.
Article10. Aboul-Fotouh GI, Zickri MB, Metwally HG, Ibrahim IR, Kamar SS, Sakr W. Therapeutic effect of adipose derived stem cells versus atorvastatin on amiodarone induced lung injury in male rat. Int J Stem Cells. 2015; 8:170–180. DOI: 10.15283/ijsc.2015.8.2.170. PMID: 26634065. PMCID: 4651281.
Article11. Takehara Y, Yabuuchi A, Ezoe K, Kuroda T, Yamadera R, Sano C, Murata N, Aida T, Nakama K, Aono F, Aoyama N, Kato K, Kato O. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest. 2013; 93:181–193. DOI: 10.1038/labinvest.2012.167. PMCID: 3561594.
Article12. Espina M, Jülke H, Brehm W, Ribitsch I, Winter K, Delling U. Evaluation of transport conditions for autologous bone marrow-derived mesenchymal stromal cells for therapeutic application in horses. PeerJ. 2016; 4:e1773. DOI: 10.7717/peerj.1773. PMID: 27019778. PMCID: 4806605.
Article13. Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006; 326:725–736. DOI: 10.1007/s00441-006-0270-9. PMID: 16906419.
Article14. Ude CC, Shamsul BS, Ng MH, Chen HC, Norhamdan MY, Aminuddin BS, Ruszymah BH. Bone marrow and adipose stem cells can be tracked with PKH26 until post staining passage 6 in in vitro and in vivo. Tissue Cell. 2012; 44:156–163. DOI: 10.1016/j.tice.2012.02.001. PMID: 22402173.
Article15. Tupling AR, Bombardier E, Gupta SC, Hussain D, Vigna C, Bloemberg D, Quadrilatero J, Trivieri MG, Babu GJ, Backx PH, Periasamy M, MacLennan DH, Gramolini AO. Enhanced Ca2+ transport and muscle relaxation in skeletal muscle from sarcolipin-null mice. Am J Physiol Cell Physiol. 2011; 301:C841–C849. DOI: 10.1152/ajpcell.00409.2010. PMID: 21697544. PMCID: 3654932.16. Mars T, Strazisar M, Mis K, Kotnik N, Pegan K, Lojk J, Grubic Z, Pavlin M. Electrotransfection and lipofection show comparable efficiency for in vitro gene delivery of primary human myoblasts. J Membr Biol. 2015; 248:273–283. DOI: 10.1007/s00232-014-9766-5.
Article17. Gruber SJ, Cornea RL, Li J, Peterson KC, Schaaf TM, Gillispie GD, Dahl R, Zsebo KM, Robia SL, Thomas DD. Discovery of enzyme modulators via high-throughput time-resolved FRET in living cells. J Biomol Screen. 2014; 19:215–222. DOI: 10.1177/1087057113510740. PMID: 24436077. PMCID: 4013825.
Article18. Shewade YM, Umrani M, Bhonde RR. Large-scale isolation of islets by tissue culture of adult mouse pancreas. Transplant Proc. 1999; 31:1721–1723. DOI: 10.1016/S0041-1345(99)00077-9. PMID: 10331051.
Article19. Aziz MT, El-Asmar MF, Rezq AM, Wassef MA, Fouad H, Roshdy NK, Ahmed HH, Rashed LA, Sabry D, Taha FM, Hassouna A. Effects of a novel curcumin derivative on insulin synthesis and secretion in streptozotocin-treated rat pancreatic islets in vitro. Chin Med. 2014; 9:3. DOI: 10.1186/1749-8546-9-3. PMID: 24422903. PMCID: 3896850.
Article20. Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006; 290:E1339–E1346. DOI: 10.1152/ajpendo.00487.2005. PMID: 16434553.
Article21. Golshan Iranpour F, Kheiri S. Coadministration of calcium chloride with lead acetate can improve motility of cauda epididymal spermatozoa in Swiss white mice. Int J Reprod Biomed (Yazd). 2016; 14:141–144. DOI: 10.29252/ijrm.14.2.141.
Article22. Hunter EE. Practical electron microscopy A Beginner’s illustrated guide. 2nd ed. Cambridge, N Y, Melbourne, Madrid, Cape Town, Singapore, Sao Paulo, Delhi and UK: Cambridge university press;1993. p. 1–70.23. Bancroft JD, Gamble M. Connective tissue stains. Theory and Practice of Histological Techniques. 6th ed. Edinburgh, London, Oxford, New York, Philadelphia, St Louis, Sydney and Toronto: Elsevier Health Sciences, Churchill Livingstone;2008. p. 150.24. Steinert AF, Kunz M, Prager P, Göbel S, Klein-Hitpass L, Ebert R, Nöth U, Jakob F, Gohlke F. Characterization of bursa subacromialis-derived mesenchymal stem cells. Stem Cell Res Ther. 2015; 6:114. DOI: 10.1186/s13287-015-0104-3. PMID: 26036250. PMCID: 4479225.
Article25. Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell. 2013; 153:747–758. DOI: 10.1016/j.cell.2013.04.008. PMID: 23623304. PMCID: 3756510.
Article26. Pu T, Guo P, Qiu Y, Chen S, Yang L, Sun L, Ye F, Bu H. Quantitative real-time polymerase chain reaction is an alternative method for the detection of HER-2 amplification in formalin-fixed paraffin-embedded breast cancer samples. Int J Clin Exp Pathol. 2015; 8:10565–10574. PMID: 26617766. PMCID: 4637581.27. Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010; 19:237–270. DOI: 10.1177/0962280209105014.
Article28. de Senna PN, Bagatini PB, Galland F, Bobermin L, do Nascimento PS, Nardin P, Tramontina AC, Gonçalves CA, Achaval M, Xavier LL. Physical exercise reverses spatial memory deficit and induces hippocampal astrocyte plasticity in diabetic rats. Brain Res. 2017; 1655:242–251. DOI: 10.1016/j.brainres.2016.10.024.
Article29. Zheng S, Zhao M, Wu Y, Wang Z, Ren Y. Suppression of pancreatic beta cell apoptosis by Danzhi Jiangtang capsule contributes to the attenuation of type 1 diabetes in rats. BMC Complement Altern Med. 2016; 16:31. DOI: 10.1186/s12906-016-0993-4. PMID: 26819084. PMCID: 4729167.
Article30. Martinov T, Spanier JA, Pauken KE, Fife BT. PD-1 pathway- mediated regulation of islet-specific CD4+ T cell subsets in autoimmune diabetes. Immunoendocrinology (Houst). 2016; 3:pii: e1164.31. Li S, Huang KJ, Wu JC, Hu MS, Sanyal M, Hu M, Longaker MT, Lorenz HP. Peripheral blood-derived mesenchymal stem cells: candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl Med. 2015; 4:359–368. DOI: 10.5966/sctm.2014-0150. PMID: 25742693. PMCID: 4367504.
Article32. Vono R, Fuoco C, Testa S, Pirrò S, Maselli D, Ferland McCollough D, Sangalli E, Pintus G, Giordo R, Finzi G, Sessa F, Cardani R, Gotti A, Losa S, Cesareni G, Rizzi R, Bearzi C, Cannata S, Spinetti G, Gargioli C, Madeddu P. Activation of the pro-oxidant PKCβII-p66Shc signaling pathway contributes to pericyte dysfunction in skeletal muscles of patients with diabetes with critical limb ischemia. Diabetes. 2016; 65:3691–3704. DOI: 10.2337/db16-0248. PMID: 27600065.
Article33. Ahn C, Kang JH, Jeung EB. Calcium homeostasis in diabetes mellitus. J Vet Sci. 2017; 18:261–266. DOI: 10.4142/jvs.2017.18.3.261. PMID: 28927245. PMCID: 5639077.
Article34. Li W, Du SN, Shi MJ, Sun ZZ. Spontaneous and transient predinner hyperglycemia in some patients with diabetes: Dusk phenomenon. Medicine (Baltimore). 2016; 95:e5440. DOI: 10.1097/MD.0000000000005440.35. Gerace D, Martiniello-Wilks R, Nassif NT, Lal S, Steptoe R, Simpson AM. CRISPR-targeted genome editing of mesenchymal stem cell-derived therapies for type 1 diabetes: a path to clinical success? Stem Cell Res Ther. 2017; 8:62. DOI: 10.1186/s13287-017-0511-8. PMID: 28279194. PMCID: 5345178.
Article36. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016; 7:125. DOI: 10.1186/s13287-016-0363-7. PMID: 27581859. PMCID: 5007684.
Article37. Liu HY, Chen CC, Lin YY, Chen YJ, Liu BH, Wong SC, Wu CY, Chang YT, Chou HE, Ding ST. Chitosan-assisted differentiation of porcine adipose tissue-derived stem cells into glucose-responsive insulin-secreting clusters. PLoS One. 2017; 12:e0172922. DOI: 10.1371/journal.pone.0172922. PMID: 28253305. PMCID: 5333835.
Article38. Ouyang Z, Li W, Meng Q, Zhang Q, Wang X, Elgehama A, Wu X, Shen Y, Sun Y, Wu X, Xu Q. A natural compound jaceosidin ameliorates endoplasmic reticulum stress and insulin resistance via upregulation of SERCA2b. Biomed Pharmacother. 2017; 89:1286–1296. DOI: 10.1016/j.biopha.2017.03.023. PMID: 28320096.
Article39. Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm Sin B. 2017; 7:3–17. DOI: 10.1016/j.apsb.2016.11.001. PMID: 28119804. PMCID: 5237760.
Article40. Jian L, Ling-Peng W. GW27-e0977 The research of L type calcium channel in Turn SERCA 2a genetic model for acute atrial fibrillation atrial muscle cell. J Am Coll Cardiol. 2016; 68(16 Suppl):C35. DOI: 10.1016/j.jacc.2016.07.128.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem Cell Therapy for Type 1 Diabetes Mellitus
- Adult Stem Cells: Beyond Regenerative Tool, More as a Bio-Marker in Obesity and Diabetes
- Current Concepts of Stem Cell Therapy
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy
- Stem Cell Properties of Therapeutic Potential