Diabetes Metab J.
2019 Dec;43(6):744-751. 10.4093/dmj.2019.0175.
Adult Stem Cells: Beyond Regenerative Tool, More as a Bio-Marker in Obesity and Diabetes
- Affiliations
-
- 1Division of Endocrinology, Department of Medicine, The George Washington University, Washington, DC, USA. ssen1@gwu.edu
- KMID: 2466495
- DOI: http://doi.org/10.4093/dmj.2019.0175
Abstract
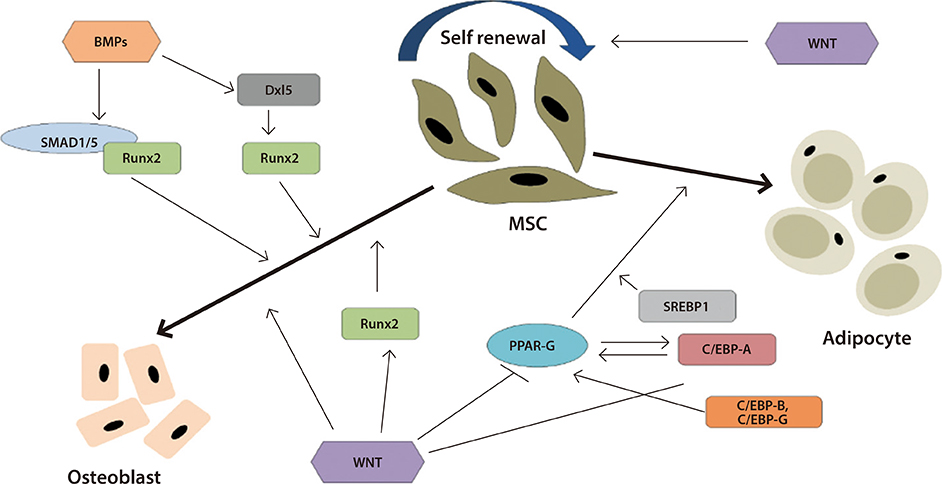
- Obesity, diabetes, and cardiovascular diseases are increasing rapidly worldwide and it is therefore important to know the effect of exercise and medications for diabetes and obesity on adult stem cells. Adult stem cells play a major role in remodeling and tissue regeneration. In this review we will focus mainly on two adult stem/progenitor cells such as endothelial progenitor cells and mesenchymal stromal cells in relation to aerobic exercise and diabetes medications, both of which can alter the course of regeneration and tissue remodelling. These two adult precursor and stem cells are easily obtained from peripheral blood or adipose tissue depots, as the case may be and are precursors to endothelium and mesenchymal tissue (fat, bone, muscle, and cartilage). They both are key players in maintenance of cardiovascular and metabolic homeostasis and can act also as useful biomarkers.
MeSH Terms
Figure
Reference
-
1. National Institute of Diabetes and Digestive and Kidney Diseases: Overweight and obesity statistics, 2014. cited 2019 Nov 20. Available from: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity.2. World Health Organization: Diabetes fact sheet 2017. cited 2019 Nov 20. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/.3. Centers for Disease Control and Prevention: High blood pressure facts 2016. cited 2019 Nov 20. Available from: https://www.cdc.gov/bloodpressure/facts.htm.4. Centers for Disease Control and Prevention: Stroke fact sheet 2017. cited 2019 Nov 20. Available from: https://www.cdc.gov/stroke/facts.htm.5. Centers for Disease Control and Prevention: Heart disease fact 2017. cited 2019 Nov 20. Available from: https://www.cdc.gov/heartdisease/facts.htm.6. Centers for Disease Control and Prevention: National diabetes statistics report 2017. cited 2019 Nov 20. Available from: https://www.cdc.gov/diabetes/data/statistics/statistics-report.html.7. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013; 36:1033–1046.8. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403.9. Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, Park SO, Hur KY, Chon S, Moon MK, Kim NH, Kim SY, Rhee SY, Lee KW, Kim JH, Rhee EJ, Chun S, Yu SH, Kim DJ, Kwon HS, Park KS. Committee of Clinical Practice Guidelines. Korean Diabetes Association. 2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019; 43:398–406.10. Kokkinos P, Narayan P. Chapter 4, Effect of exercise on adult stem cells. Cardiorespiratory fitness in cardiometabolic diseases. Cham: Springer Nature Publications;2019. p. 49–56.11. Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011; 9:12.12. Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012; 2:a006692.13. Van Craenenbroeck EM, Conraads VM. Endothelial progenitor cells in vascular health: focus on lifestyle. Microvasc Res. 2010; 79:184–192.14. Boppart MD, De Lisio M, Witkowski S. Exercise and stem cells. Prog Mol Biol Transl Sci. 2015; 135:423–456.15. Francois ME, Pistawka KJ, Halperin FA, Little JP. Cardiovascular benefits of combined interval training and post-exercise nutrition in type 2 diabetes. J Diabetes Complications. 2018; 32:226–233.16. Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004; 109:220–226.17. Steiner S, Niessner A, Ziegler S, Richter B, Seidinger D, Pleiner J, Penka M, Wolzt M, Huber K, Wojta J, Minar E, Kopp CW. Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis. 2005; 181:305–310.18. Jenkins NT, Witkowski S, Spangenburg EE, Hagberg JM. Effects of acute and chronic endurance exercise on intracellular nitric oxide in putative endothelial progenitor cells: role of NAPDH oxidase. Am J Physiol Heart Circ Physiol. 2009; 297:H1798–H1805.19. Gensch C, Clever Y, Werner C, Hanhoun M, Bohm M, Laufs U. Regulation of endothelial progenitor cells by prostaglandin E1 via inhibition of apoptosis. J Mol Cell Cardiol. 2007; 42:670–677.20. Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, Tarnok A, Gielen S, Emmrich F, Schuler G, Hambrecht R. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol. 2004; 24:684–690.21. Sarto P, Balducci E, Balconi G, Fiordaliso F, Merlo L, Tuzzato G, Pappagallo GL, Frigato N, Zanocco A, Forestieri C, Azzarello G, Mazzucco A, Valenti MT, Alborino F, Noventa D, Vinante O, Pascotto P, Sartore S, Dejana E, Latini R. Effects of exercise training on endothelial progenitor cells in patients with chronic heart failure. J Card Fail. 2007; 13:701–708.22. Witkowski S, Lockard MM, Jenkins NT, Obisesan TO, Spangenburg EE, Hagberg JM. Relationship between circulating progenitor cells, vascular function and oxidative stress with long-term training and short-term detraining in older men. Clin Sci (Lond). 2010; 118:303–311.23. Hoetzer GL, Van Guilder GP, Irmiger HM, Keith RS, Stauffer BL, DeSouza CA. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol (1985). 2007; 102:847–852.24. Thijssen DH, Vos JB, Verseyden C, van Zonneveld AJ, Smits P, Sweep FC, Hopman MT, de Boer HC. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell. 2006; 5:495–503.25. Sen S, Witkowski S, Lagoy A, Islam AM. A six-week home exercise program improves endothelial function and CD34+ circulating progenitor cells in patients with pre-diabetes. J Endo crinol Metab. 2015; 5:163–171.26. Van Craenenbroeck EM, Bruyndonckx L, Van Berckelaer C, Hoymans VY, Vrints CJ, Conraads VM. The effect of acute exercise on endothelial progenitor cells is attenuated in chronic heart failure. Eur J Appl Physiol. 2011; 111:2375–2379.27. Ajijola OA, Dong C, Herderick EE, Ma Q, Goldschmidt-Clermont PJ, Yan Z. Voluntary running suppresses proinflammatory cytokines and bone marrow endothelial progenitor cell levels in apolipoprotein-E-deficient mice. Antioxid Redox Signal. 2009; 11:15–23.28. Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Groger M, Fialka-Moser V, Gschwandtner M, Koppensteiner R, Steiner S. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis. 2011; 217:240–248.29. Van Craenenbroeck EM, Beckers PJ, Possemiers NM, Wuyts K, Frederix G, Hoymans VY, Wuyts F, Paelinck BP, Vrints CJ, Conraads VM. Exercise acutely reverses dysfunction of circulating angiogenic cells in chronic heart failure. Eur Heart J. 2010; 31:1924–1934.30. Rehman J, Li J, Parvathaneni L, Karlsson G, Panchal VR, Temm CJ, Mahenthiran J, March KL. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol. 2004; 43:2314–2318.31. Fernandes T, Nakamuta JS, Magalhaes FC, Roque FR, Lavini-Ramos C, Schettert IT, Coelho V, Krieger JE, Oliveira EM. Exercise training restores the endothelial progenitor cells number and function in hypertension: implications for angiogenesis. J Hypertens. 2012; 30:2133–2143.32. Wahl P, Brixius K, Bloch W. Exercise-induced stem cell activation and its implication for cardiovascular and skeletal muscle regeneration. Minim Invasive Ther Allied Technol. 2008; 17:91–99.33. Laufs U, Urhausen A, Werner N, Scharhag J, Heitz A, Kissner G, Bohm M, Kindermann W, Nickenig G. Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil. 2005; 12:407–414.34. Dore FJ, Domingues CC, Ahmadi N, Kundu N, Kropotova Y, Houston S, Rouphael C, Mammadova A, Witkin L, Khiyami A, Amdur RL, Sen S. The synergistic effects of saxagliptin and metformin on CD34+ endothelial progenitor cells in early type 2 diabetes patients: a randomized clinical trial. Cardiovasc Diabetol. 2018; 17:65.35. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001; 7:211–228.36. Zanini C, Bruno S, Mandili G, Baci D, Cerutti F, Cenacchi G, Izzi L, Camussi G, Forni M. Differentiation of mesenchymal stem cells derived from pancreatic islets and bone marrow into islet-like cell phenotype. PLoS One. 2011; 6:e28175.37. Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013; 13:856–867.38. Jamal F. Chapter 5, Genetic modification of stem cells in diabetes and obesity. Genetic engineering: an insight into the strategies and applications. London: InTech;2016. p. 75–84.39. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317.40. Schmidt A, Bierwirth S, Weber S, Platen P, Schinkothe T, Bloch W. Short intensive exercise increases the migratory activity of mesenchymal stem cells. Br J Sports Med. 2009; 43:195–198.41. Emmons R, Niemiro GM, Owolabi O, De Lisio M. Acute exercise mobilizes hematopoietic stem and progenitor cells and alters the mesenchymal stromal cell secretome. J Appl Physiol (1985). 2016; 120:624–632.42. Shin MS, Park HK, Kim TW, Ji ES, Lee JM, Choi HS, Kim MY, Kim YP. Neuroprotective effects of bone marrow stromal cell transplantation in combination with treadmill exercise following traumatic brain injury. Int Neurourol J. 2016; 20:Suppl 1. S49–S56.43. Zhang YX, Yuan MZ, Cheng L, Lin LZ, Du HW, Chen RH, Liu N. Treadmill exercise enhances therapeutic potency of transplanted bone mesenchymal stem cells in cerebral ischemic rats via anti-apoptotic effects. BMC Neurosci. 2015; 16:56.44. Gibbs N, Diamond R, Sekyere EO, Thomas WD. Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: a case series. J Pain Res. 2015; 8:799–806.45. Aoyama T, Fujita Y, Madoba K, Nankaku M, Yamada M, Tomita M, Goto K, Ikeguchi R, Kakinoki R, Matsuda S, Nakamura T, Toguchida J. Rehabilitation program after mesenchymal stromal cell transplantation augmented by vascularized bone grafts for idiopathic osteonecrosis of the femoral head: a preliminary study. Arch Phys Med Rehabil. 2015; 96:532–539.46. Li R, Liang L, Dou Y, Huang Z, Mo H, Wang Y, Yu B. Mechanical strain regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. Biomed Res Int. 2015; 2015:873251.47. Liu SY, He YB, Deng SY, Zhu WT, Xu SY, Ni GX. Exercise affects biological characteristics of mesenchymal stromal cells derived from bone marrow and adipose tissue. Int Orthop. 2017; 41:1199–1209.48. Kundu N, Domingues CC, Nylen ES, Paal E, Kokkinos P, Sen S. Endothelium-derived factors influence differentiation of fat-derived stromal cells post-exercise in subjects with prediabetes. Metab Syndr Relat Disord. 2019; 17:314–322.49. Cook D, Genever P. Regulation of mesenchymal stem cell differentiation. Adv Exp Med Biol. 2013; 786:213–229.50. Maredziak M, Smieszek A, Chrząstek K, Basinska K, Marycz K. Physical activity increases the total number of bone-marrow-derived mesenchymal stem cells, enhances their osteogenic potential, and inhibits their adipogenic properties. Stem Cells Int. 2015; 2015:379093.51. Yamaguchi S, Aoyama T, Ito A, Nagai M, Iijima H, Tajino J, Zhang X, Kiyan W, Kuroki H. The effect of exercise on the early stages of mesenchymal stromal cell-induced cartilage repair in a rat osteochondral defect model. PLoS One. 2016; 11:e0151580.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell Replacement and Regeneration Therapy for Diabetes
- Delivering Factors for Reprogramming a Somatic Cell to Pluripotency
- Hematopoietic Stem Cells and Their Roles in Tissue Regeneration
- Stem cells: general information and perspectives
- Induced pluripotent stem cells and personalized medicine: current progress and future perspectives