Nutr Res Pract.
2018 Feb;12(1):41-46. 10.4162/nrp.2018.12.1.41.
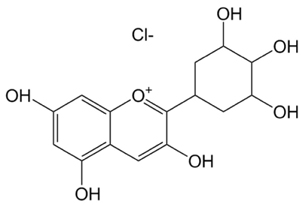
Radioprotective effects of delphinidin on normal human lung cells against proton beam exposure
- Affiliations
-
- 1Department of Medical Science, Konyang University, 158 Gwanjeodong-ro, Seo-gu, Daejeon 35365, Korea. bskang@konyang.ac.kr
- 2Department of Food & Nutrition, Hyejeon College, Chungnam 32244, Korea.
- KMID: 2429017
- DOI: http://doi.org/10.4162/nrp.2018.12.1.41
Abstract
- BACKGROUND/OBJECTIVES
Exposure of the normal lung tissue around the cancerous tumor during radiotherapy causes serious side effects such as pneumonitis and pulmonary fibrosis. Radioprotectors used during cancer radiotherapy could protect the patient from side effects induced by radiation injury of the normal tissue. Delphinidin has strong antioxidant properties, and it works as the driving force of a radioprotective effect by scavenging radiation-induced reactive oxygen species (ROS). However, no studies have been conducted on the radioprotective effect of delphinidin against high linear energy transfer radiation. Therefore, this study was undertaken to evaluate the radioprotective effects of delphinidin on human lung cells against a proton beam.
MATERIALS/METHODS
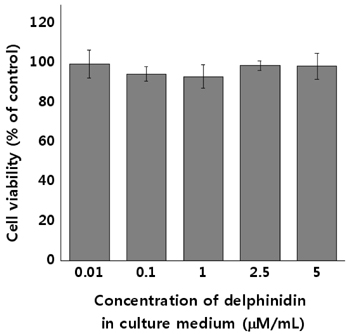
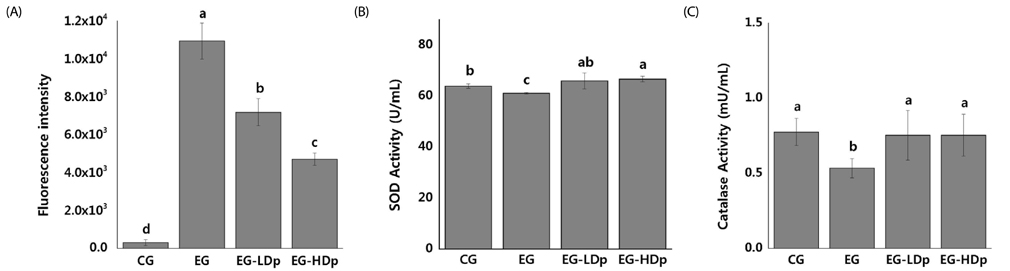
Normal human lung cells (HEL 299 cells) were used for in vitro experiments. The 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay assessed the cytotoxicity of delphinidin and cell viability. The expression of radiation induced cellular ROS was measured by the 2"²-7"²-dicholordihydrofluorescein diacetate assay. Superoxide dismutase activity assay and catalase activity assay were used for evaluating the activity of corresponding enzymes. In addition, radioprotective effects on DNA damage-induced cellular apoptosis were evaluated by Western blot assay.
RESULTS
Experimental analysis, including cell survival assay, MTT assay, and Western blot assay, revealed the radioprotective effects of delphinidin. These include restoring the activities of antioxidant enzymes of damaged cells, increase in the levels of pro-survival protein, and decrease of pro-apoptosis proteins. The results from different experiments were compatible with each to provide a substantial conclusion.
CONCLUSION
Low concentration (2.5 µM/mL) of delphinidin administration prior to radiation exposure was radioprotective against a low dose of proton beam exposure. Hence, delphinidin is a promising shielding agent against radiation, protecting the normal tissues around a cancerous tumor, which are unintentionally exposed to low doses of radiation during proton therapy.
MeSH Terms
-
Apoptosis
Blotting, Western
Catalase
Cell Survival
DNA
Humans*
In Vitro Techniques
Linear Energy Transfer
Lung*
Pneumonia
Proton Therapy
Protons*
Pulmonary Fibrosis
Radiation Exposure
Radiation Injuries
Radiotherapy
Reactive Oxygen Species
Superoxide Dismutase
Catalase
DNA
Protons
Reactive Oxygen Species
Superoxide Dismutase
Figure
Reference
-
1. Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005; 104:1129–1137.2. Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013; 14:1347–1356.
Article3. Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today. 2007; 12:794–805.
Article4. Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 2010; 15:360–371.
Article5. Kamran MZ, Ranjan A, Kaur N, Sur S, Tandon V. Radioprotective agents: strategies and translational advances. Med Res Rev. 2016; 36:461–493.
Article6. Lim JY, Kim OK, Lee J, Lee MJ, Kang N, Hwang JK. Protective effect of the standardized green tea seed extract on UVB-induced skin photoaging in hairless mice. Nutr Res Pract. 2014; 8:398–403.
Article7. Seo EY. Delphinidin inhibits cell proliferation and induces apoptosis in MDA-MB-231 human breast cancer cell lines. J Nutr Health. 2013; 46:503–510.
Article8. Kim SN, Kim MR, Cho SM, Kim SY, Kim JB, Cho YS. Antioxidant activities and determination of phenolic compounds isolated from oriental plums (Soldam, Oishiwase and Formosa). Nutr Res Pract. 2012; 6:277–285.
Article9. Kim YH, Kim DS, Woo SS, Kim HH, Lee YS, Kim HS, Ko KO, Lee SK. Antioxidant activity and cytotoxicity on human cancer cells of anthocyanin extracted from black soybean. Korean J Crop Sci. 2008; 53:407–412.10. Choung MG, Lim JD. Antioxidant, anticancer and immune activation of anthocyanin fraction from Rubus coreanus Miquel fruits (Bokbunja). Korean J Med Crop Sci. 2012; 20:259–269.
Article11. Kim YK, Yoon HH, Lee YD, Youn DY, Ha TJ, Kim HS, Lee JH. Anthocyanin extracts from black soybean (Glycine Max L.) protect human glial cells against oxygen-glucose deprivation by promoting autophagy. Biomol Ther (Seoul). 2012; 20:68–74.
Article12. Choi EY. Protective effect of anthocyanin-rich bilberry (Vaccinium myrtillus L.) extract against Doxorubicin-induced cardiotoxicity [doctor's thesis]. Seoul: Ewha Womans University;2009.13. Zhao JG, Yan QQ, Lu LZ, Zhang YQ. In vivo antioxidant, hypoglycemic, and anti-tumor activities of anthocyanin extracts from purple sweet potato. Nutr Res Pract. 2013; 7:359–365.
Article14. Watson RR, Schönlau F. Nutraceutical and antioxidant effects of a delphinidin-rich maqui berry extract Delphinol®: a review. Minerva Cardioangiol. 2015; 63:1–12.15. Jhin C, Hwang KT. Prediction of radical scavenging activities of anthocyanins applying adaptive neuro-fuzzy inference system (ANFIS) with quantum chemical descriptors. Int J Mol Sci. 2014; 15:14715–14727.
Article16. Zhao H, Wang Z, Ma F, Yang X, Cheng C, Yao L. Protective effect of anthocyanin from Lonicera Caerulea var. Edulis on radiation-induced damage in mice. Int J Mol Sci. 2012; 13:11773–11782.
Article17. Fan ZL, Wang ZY, Zuo LL, Tian SQ. Protective effect of anthocyanins from lingonberry on radiation-induced damages. Int J Environ Res Public Health. 2012; 9:4732–4743.
Article18. Liu W, Lu X, He G, Gao X, Li M, Wu J, Li Z, Wu J, Wang J, Luo C. Cytosolic protection against ultraviolet induced DNA damage by blueberry anthocyanins and anthocyanidins in hepatocarcinoma HepG2 cells. Biotechnol Lett. 2013; 35:491–498.
Article19. Alan Mitteer R, Wang Y, Shah J, Gordon S, Fager M, Butter PP, Kim HJ, Guardiola-Salmeron C, Carabe-Fernandez A, Fan Y. Proton beam radiation induces DNA damage and cell apoptosis in glioma stem cells through reactive oxygen species. Sci Rep. 2015; 5:13961.
Article20. Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, Minor L. Assay guidance manual: cell viability assays [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences;2013. cited 2004 September 7. Available from: https://www.ncbi.nlm.nih.gov/books/NBK144065/.21. Degli Esposti M. Measuring mitochondrial reactive oxygen species. Methods. 2002; 26:335–340.
Article22. BioVision (USA). Superoxide dismutase (SOD) activity colorimetric assay kit [Internet]. Milpitas (CA): BioVision;2017. cited 2017 September 30. Available from: https://www.biovision.com/documentation/datasheets/K335.pdf.23. BioVision (USA). Catalase activity colorimetric/fluorometric assay kit [Internet]. Milpitas (CA): BioVision;2017. cited 2017 September 30. Available from: https://www.biovision.com/documentation/datasheets/K773.pdf.24. Mahmood T, Yang PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci. 2012; 4:429–434.
Article25. Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012; 327:48–60.
Article26. Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000; 32:157–170.
Article27. Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007; 74:324–329.
Article28. Chiang AN, Wu HL, Yeh HI, Chu CS, Lin HC, Lee WC. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids. 2006; 41:797–803.
Article29. Mansour HH. Protective role of carnitine ester against radiation-induced oxidative stress in rats. Pharmacol Res. 2006; 54:165–171.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Delphinidin enhances radio-therapeutic effects via autophagyinduction and JNK/MAPK pathway activation in non-small celllung cancer
- Delphinidin inhibits cell proliferation and induces apoptosis in MDA-MB-231 human breast cancer cell lines
- A Pilot Study of the Scanning Beam Quality Assurance Using Machine Log Files in Proton Beam Therapy
- Suppression of β-catenin Signaling Pathway in Human Prostate Cancer PC3 Cells by Delphinidin
- Recent Updates on Radiation Therapy for Pediatric Optic Pathway Glioma