Nutr Res Pract.
2018 Feb;12(1):3-12. 10.4162/nrp.2018.12.1.3.
Sageretia thea fruit extracts rich in methyl linoleate and methyl linolenate downregulate melanogenesis via the Akt/GSK3β signaling pathway
- Affiliations
-
- 1Faculty of Biotechnology, College of Applied Life Sciences, SARI, Jeju National University, 102, Jejudaehak-ro, Jeju-si, Jeju 63243, Korea. somikim@jejunu.ac.kr
- 2Subtropical Horticulture Research Institute, Jeju National University, Jeju 63243, Korea.
- 3Subtropical Tropical Organism Gene Bank, Jeju National University, Jeju 63243, Korea.
- KMID: 2429013
- DOI: http://doi.org/10.4162/nrp.2018.12.1.3
Abstract
- BACKGROUND/OBJECTIVES
Sageretia thea is traditionally used as a medicinal herb to treat various diseases, including skin disorders, in China and Korea. This study evaluated the inhibitory effect of Sageretia thea fruit on melanogenesis and its underlying mechanisms in B16F10 mouse melanoma cells. The active chemical compounds in anti-melanogenesis were determined in Sageretia thea.
MATERIALS/METHODS
Solvent fractions from the crude extract were investigated for anti-melanogenic activities. These activities and the mechanism of anti-melanogenesis in B16F10 cells were examined by determining melanin content and tyrosinase activity, and by performing western blotting.
RESULTS
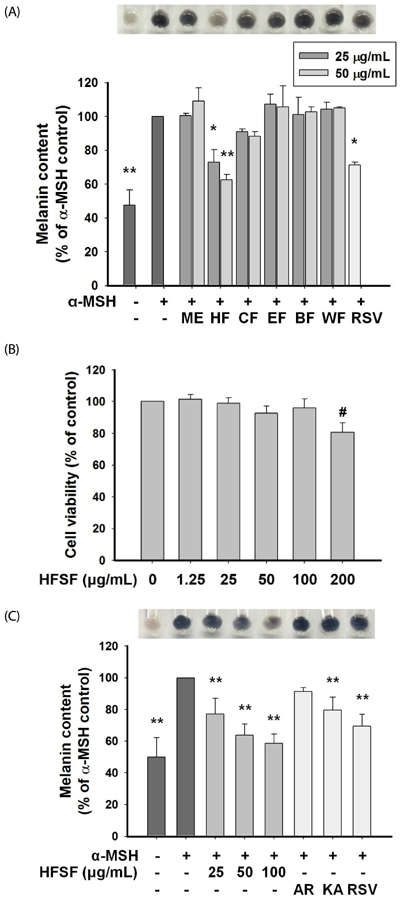
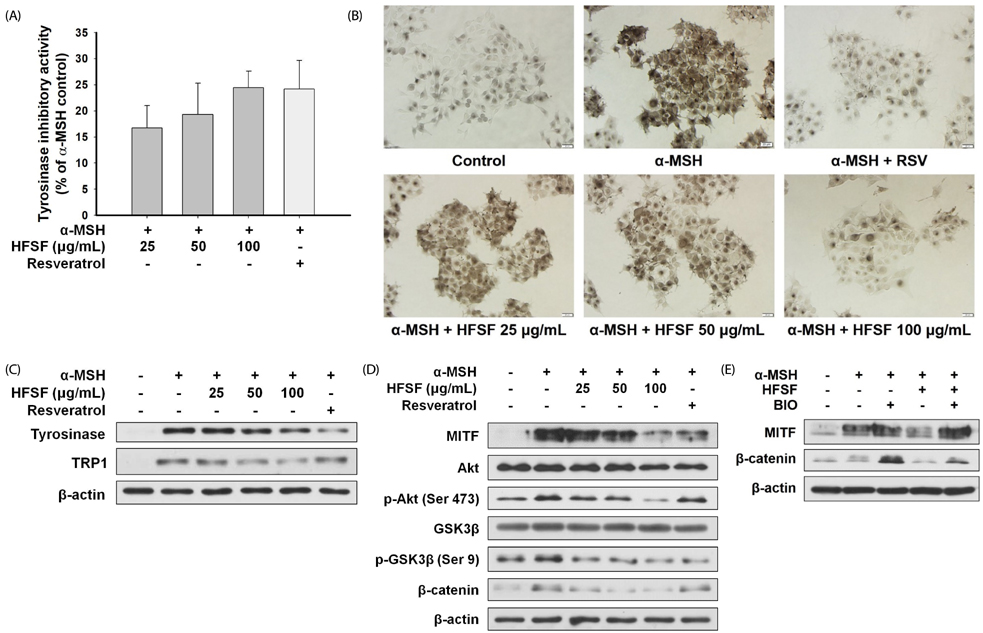
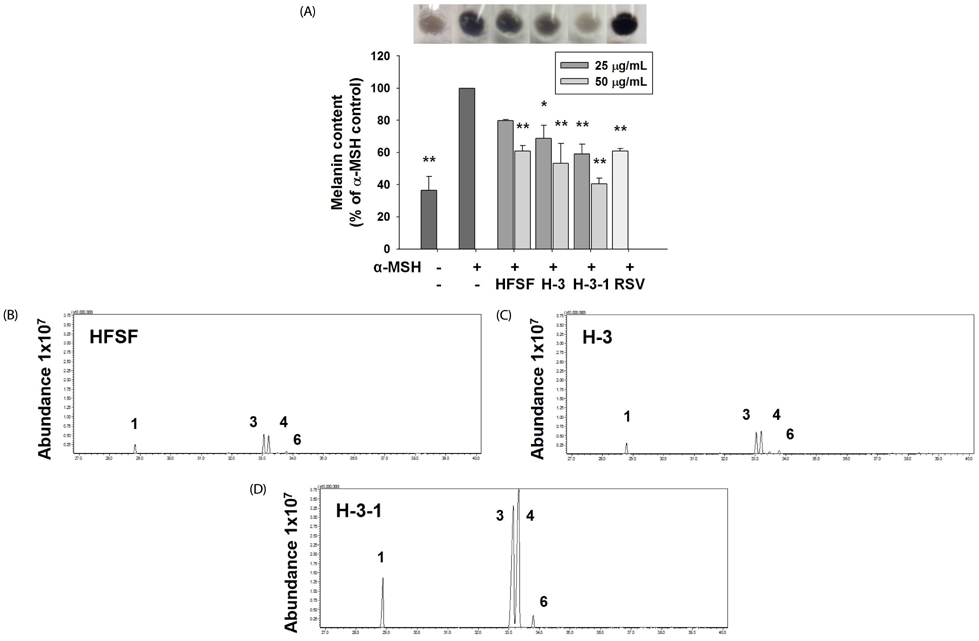
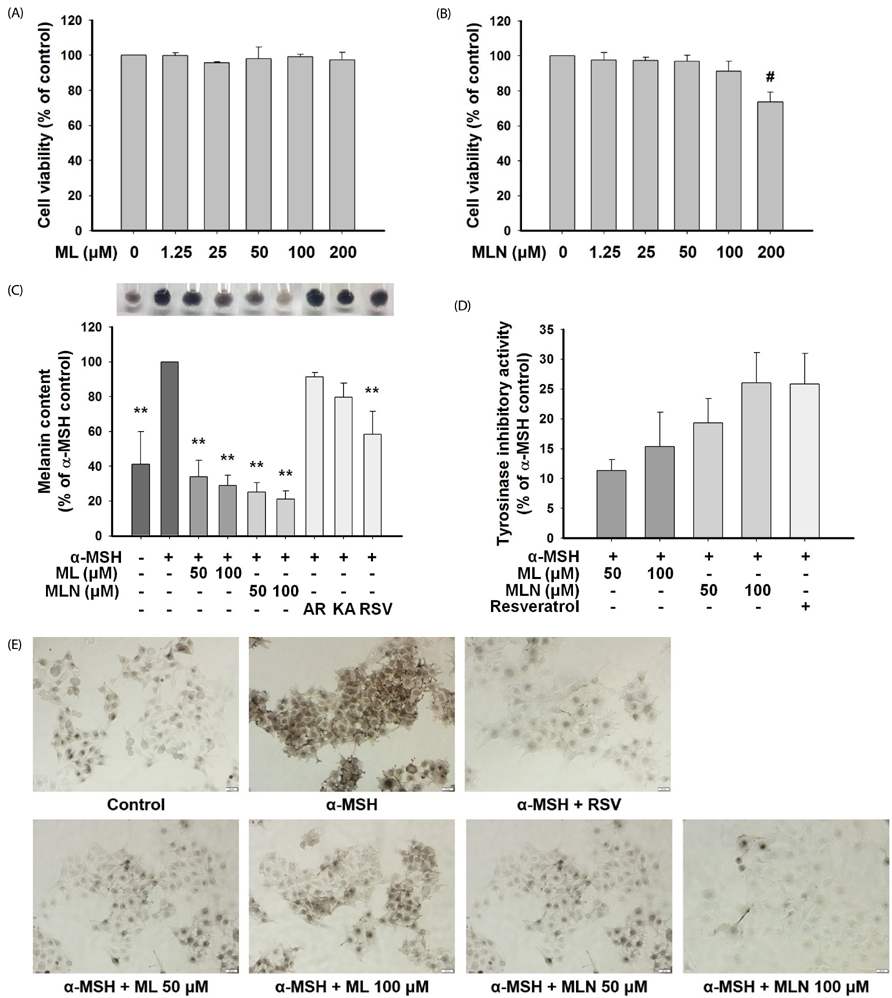
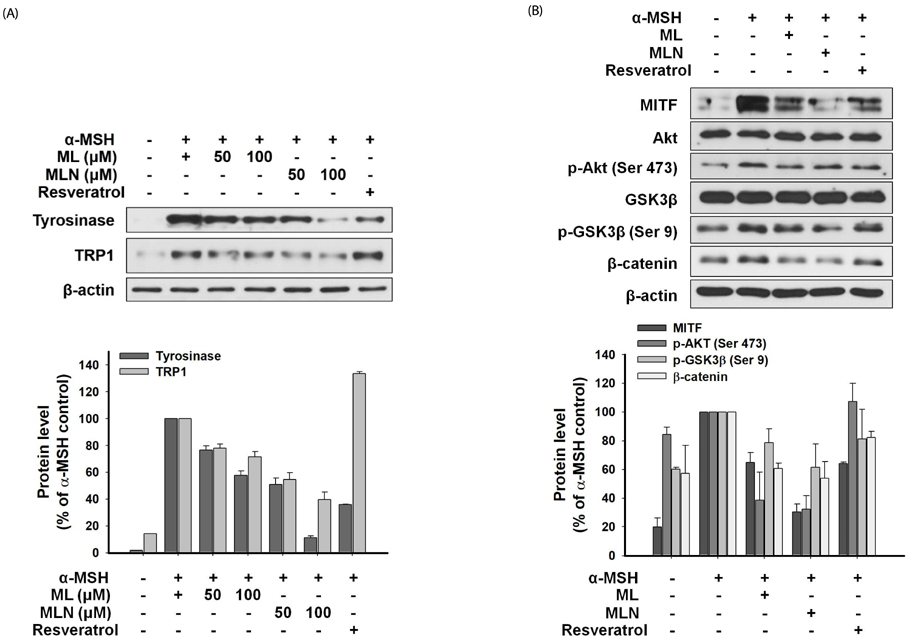
The n-hexane fraction of Sageretia thea fruit (HFSF) exhibited significant anti-melanogenic activity among the various solvent fractions without reducing viability of B16F10 cells. The HFSF suppressed the expression of tyrosinase and tyrosinase-related protein 1 (TRP1). The reduction of microphthalmia-associated transcription factor (MITF) expression by the HFSF was mediated by the Akt/glycogen synthase kinase 3 beta (GSK3β) signaling pathway, which promotes the reduction of β-catenin. Treatment with the GSK3β inhibitor 6-bromoindirubin-3'-oxime (BIO) restored HFSF-induced inhibition of MITF expression. The HFSF bioactive constituents responsible for anti-melanogenic activity were identified by bioassay-guided fractionation and gas chromatography-mass spectrometry analysis as methyl linoleate and methyl linolenate.
CONCLUSIONS
These results indicate that HFSF and its constituents, methyl linoleate and methyl linolenate, could be used as whitening agents in cosmetics and have potential for treating hyperpigmentation disorders in the clinic.
MeSH Terms
-
alpha-Linolenic Acid*
Animals
Bleaching Agents
Blotting, Western
Camellia*
China
Fruit*
Gas Chromatography-Mass Spectrometry
Hyperpigmentation
Korea
Linoleic Acid*
Melanins
Melanoma
Mice
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Phosphotransferases
Plants, Medicinal
Skin
Bleaching Agents
Linoleic Acid
Melanins
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Phosphotransferases
alpha-Linolenic Acid
Figure
Reference
-
1. Swope VB, Jameson JA, McFarland KL, Supp DM, Miller WE, McGraw DW, Patel MA, Nix MA, Millhauser GL, Babcock GF, Abdel-Malek ZA. Defining MC1R regulation in human melanocytes by its agonist alpha-melanocortin and antagonists agouti signaling protein and beta-defensin 3. J Invest Dermatol. 2012; 132:2255–2262.
Article2. Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007; 128:853–864.
Article3. Speeckaert R, Van Gele M, Speeckaert MM, Lambert J, van Geel N. The biology of hyperpigmentation syndromes. Pigment Cell Melanoma Res. 2014; 27:512–524.
Article4. Yatsu A, Ohbayashi N, Tamura K, Fukuda M. Syntaxin-3 is required for melanosomal localization of Tyrp1 in melanocytes. J Invest Dermatol. 2013; 133:2237–2246.
Article5. Zhang P, Liu W, Zhu C, Yuan X, Li D, Gu W, Ma H, Xie X, Gao T. Silencing of GPNMB by siRNA inhibits the formation of melanosomes in melanocytes in a MITF-independent fashion. PLoS One. 2012; 7:e42955.
Article6. Zhang Y, Helke KL, Coelho SG, Valencia JC, Hearing VJ, Sun S, Liu B, Li Z. Essential role of the molecular chaperone gp96 in regulating melanogenesis. Pigment Cell Melanoma Res. 2014; 27:82–89.
Article7. Ho H, Ganesan AK. The pleiotropic roles of autophagy regulators in melanogenesis. Pigment Cell Melanoma Res. 2011; 24:595–604.
Article8. Kim ES, Park SJ, Goh MJ, Na YJ, Jo DS, Jo YK, Shin JH, Choi ES, Lee HK, Kim JY, Jeon HB, Kim JC, Cho DH. Mitochondrial dynamics regulate melanogenesis through proteasomal degradation of MITF via ROS-ERK activation. Pigment Cell Melanoma Res. 2014; 27:1051–1062.
Article9. Beaumont KA, Smit DJ, Liu YY, Chai E, Patel MP, Millhauser GL, Smith JJ, Alewood PF, Sturm RA. Melanocortin-1 receptor-mediated signalling pathways activated by NDP-MSH and HBD3 ligands. Pigment Cell Melanoma Res. 2012; 25:370–374.
Article10. Abrisqueta M, Herraiz C, Pérez Oliva AB, Sanchez-Laorden BL, Olivares C, Jiménez-Cervantes C, García-Borrón JC. Differential and competitive regulation of human melanocortin 1 receptor signaling by beta-arrestin isoforms. J Cell Sci. 2013; 126:3724–3737.11. Khaled M, Larribere L, Bille K, Aberdam E, Ortonne JP, Ballotti R, Bertolotto C. Glycogen synthase kinase 3β is activated by cAMP and plays an active role in the regulation of melanogenesis. J Biol Chem. 2002; 277:33690–33697.
Article12. Takeda K, Takemoto C, Kobayashi I, Watanabe A, Nobukuni Y, Fisher DE, Tachibana M. Ser298 of MITF, a mutation site in Waardenburg syndrome type 2, is a phosphorylation site with functional significance. Hum Mol Genet. 2000; 9:125–132.
Article13. Bellei B, Flori E, Izzo E, Maresca V, Picardo M. GSK3beta inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell Signal. 2008; 20:1750–1761.
Article14. Zhu PY, Yin WH, Wang MR, Dang YY, Ye XY. Andrographolide suppresses melanin synthesis through Akt/GSK3beta/beta-catenin signal pathway. J Dermatol Sci. 2015; 79:74–83.
Article15. Bellei B, Pitisci A, Catricalà C, Larue L, Picardo M. Wnt/beta-catenin signaling is stimulated by alpha-melanocyte-stimulating hormone in melanoma and melanocyte cells: implication in cell differentiation. Pigment Cell Melanoma Res. 2011; 24:309–325.
Article16. Hwang E, Lee TH, Lee WJ, Shim WS, Yeo EJ, Kim S, Kim SY. A novel synthetic Piper amide derivative NED-180 inhibits hyperpigmentation by activating the PI3K and ERK pathways and by regulating Ca2+ influx via TRPM1 channels. Pigment Cell Melanoma Res. 2016; 29:81–91.
Article17. Chung KW, Jeong HO, Jang EJ, Choi YJ, Kim DH, Kim SR, Lee KJ, Lee HJ, Chun P, Byun Y, Moon HR, Chung HY. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim Biophys Acta. 2013; 1830:4752–4761.
Article18. García-Gavín J, González-Vilas D, Fernández-Redondo V, Toribio J. Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact Dermatitis. 2010; 62:63–64.
Article19. Takizawa T, Imai T, Onose J, Ueda M, Tamura T, Mitsumori K, Izumi K, Hirose M. Enhancement of hepatocarcinogenesis by kojic acid in rat two-stage models after initiation with N-bis(2-hydroxypropyl) nitrosamine or N-diethylnitrosamine. Toxicol Sci. 2004; 81:43–49.
Article20. Hong YH, Jung EY, Noh DO, Suh HJ. Physiological effects of formulation containing tannase-converted green tea extract on skin care: physical stability, collagenase, elastase, and tyrosinase activities. Integr Med Res. 2014; 3:25–33.
Article21. Chiang HM, Chien YC, Wu CH, Kuo YH, Wu WC, Pan YY, Su YH, Wen KC. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem Toxicol. 2014; 65:129–139.
Article22. Chung SK, Kim YC, Takaya Y, Terashima K, Niwa M. Novel flavonol glycoside, 7-O-methyl mearnsitrin, from Sageretia theezans and its antioxidant effect. J Agric Food Chem. 2004; 52:4664–4668.
Article23. Chung SK, Chen CY, Blumberg JB. Flavonoid-rich fraction from Sageretia theezans leaves scavenges reactive oxygen radical species and increases the resistance of low-density lipoprotein to oxidation. J Med Food. 2009; 12:1310–1315.
Article24. Hyun TK, Song SC, Song CK, Kim JS. Nutritional and nutraceutical characteristics of Sageretia theezans fruit. J Food Drug Anal. 2015; 23:742–749.
Article25. Song SC. Characteristics of Sageretia thea (Osbeck) M.C. Johnst native to Jeju Island and effects of plant growth regulator treatments on fruit quality [Ph.D. thesis]. Jeju: Jeju National University;2014.26. Akihisa T, Tochizawa S, Takahashi N, Yamamoto A, Zhang J, Kikuchi T, Fukatsu M, Tokuda H, Suzuki N. Melanogenesis-inhibitory saccharide fatty acid esters and other constituents of the fruits of Morinda citrifolia (noni). Chem Biodivers. 2012; 9:1172–1187.
Article27. Diwakar G, Rana J, Saito L, Vredeveld D, Zemaitis D, Scholten J. Inhibitory effect of a novel combination of Salvia hispanica (chia) seed and Punica granatum (pomegranate) fruit extracts on melanin production. Fitoterapia. 2014; 97:164–171.
Article28. Nam JH, Lee DU. Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J Dermatol Sci. 2016; 84:305–313.
Article29. Hosoi J, Abe E, Suda T, Kuroki T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985; 45:1474–1478.30. Newton RA, Cook AL, Roberts DW, Leonard JH, Sturm RA. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J Invest Dermatol. 2007; 127:2216–2227.
Article31. Lee EJ, Lee YS, Hwang S, Kim S, Hwang JS, Kim TY. N-(3,5-dimethylphenyl)-3-methoxybenzamide (A(3)B(5)) targets TRP-2 and inhibits melanogenesis and melanoma growth. J Invest Dermatol. 2011; 131:1701–1709.
Article32. Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T, Saito H, Takahashi K, Shibahara S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem. 2000; 275:14013–14016.
Article33. Hsiao JJ, Fisher DE. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch Biochem Biophys. 2014; 563:28–34.
Article34. Hwang I, Park JH, Park HS, Choi KA, Seol KC, Oh SI, Kang S, Hong S. Neural stem cells inhibit melanin production by activation of Wnt inhibitors. J Dermatol Sci. 2013; 72:274–283.
Article35. Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol. 1998; 8:573–581.
Article36. Routray W, Orsat V. Blueberries and their anthocyanins: factors affecting biosynthesis and properties. Compr Rev Food Sci Food Saf. 2011; 10:303–320.
Article37. Aramwit P, Bang N, Srichana T. The properties and stability of anthocyanins in mulberry fruits. Food Res Int. 2010; 43:1093–1097.
Article38. Huh S, Kim YS, Jung E, Lim J, Jung KS, Kim MO, Lee J, Park D. Melanogenesis inhibitory effect of fatty acid alkyl esters isolated from Oxalis triangularis. Biol Pharm Bull. 2010; 33:1242–1245.
Article39. Jung H, Chung H, Chang SE, Choi S, Han IO, Kang DH, Oh ES. Syndecan-2 regulates melanin synthesis via protein kinase C betaII-mediated tyrosinase activation. Pigment Cell Melanoma Res. 2014; 27:387–397.
Article40. Jiménez-Cervantes C, Martínez-Esparza M, Pérez C, Daum N, Solano F, García-Borrón JC. Inhibition of melanogenesis in response to oxidative stress: transient downregulation of melanocyte differentiation markers and possible involvement of microphthalmia transcription factor. J Cell Sci. 2001; 114:2335–2344.
Article41. Kamei Y, Otsuka Y, Abe K. Comparison of the inhibitory effects of vitamin E analogues on melanogenesis in mouse B16 melanoma cells. Cytotechnology. 2009; 59:183–190.
Article42. Sestáková B, Ondrusová L, Vachtenheim J. Cell cycle inhibitor p21/WAF1/CIP1 as a cofactor of MITF expression in melanoma cells. Pigment Cell Melanoma Res. 2010; 23:238–251.
Article43. Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3β in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000; 20:2567–2574.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ethyl linoleate inhibits α-MSH-induced melanogenesis through Akt/GSK3β/β-catenin signal pathway
- Effects of Lobetyolin, Lobetyol and Methyl linoleate on Secretion, Production and Gene Expression of MUC5AC Mucin from Airway Epithelial Cells
- Cytotoxic Effects of Gallic Acid and its Derivatives Against HIV-I-infected Microglia
- Methyl p-Hydroxycinnamate Suppresses Lipopolysaccharide-Induced Inflammatory Responses through Akt Phosphorylation in RAW264.7 Cells
- Ezrin-radixin-moesin proteins are regulated by Akt-GSK3β signaling in the rat nucleus accumbens core