Korean J Physiol Pharmacol.
2018 Jan;22(1):53-61. 10.4196/kjpp.2018.22.1.53.
Ethyl linoleate inhibits α-MSH-induced melanogenesis through Akt/GSK3β/β-catenin signal pathway
- Affiliations
-
- 1Faculty of Biotechnology, College of Applied Life Sciences, SARI, Jeju National University, Jeju 63243, Korea. somikim@jejunu.ac.kr
- 2Subtropical Tropical Organism Gene Bank, Jeju National University, Jeju 63243, Korea.
- KMID: 2398555
- DOI: http://doi.org/10.4196/kjpp.2018.22.1.53
Abstract
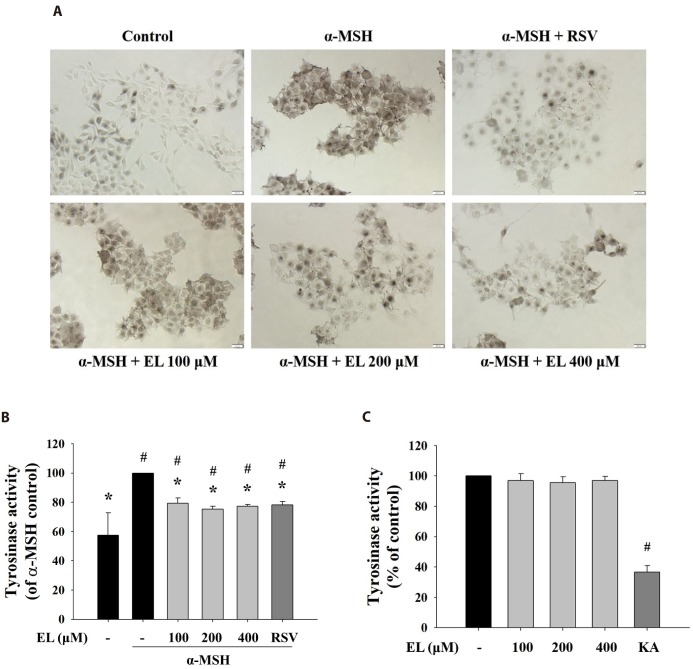
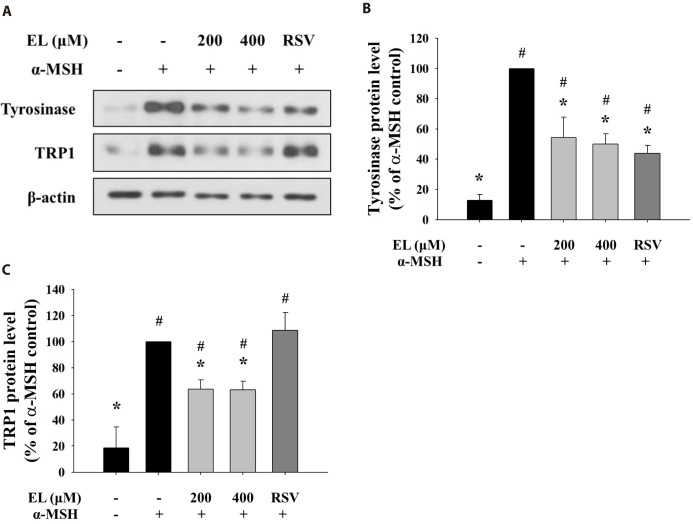
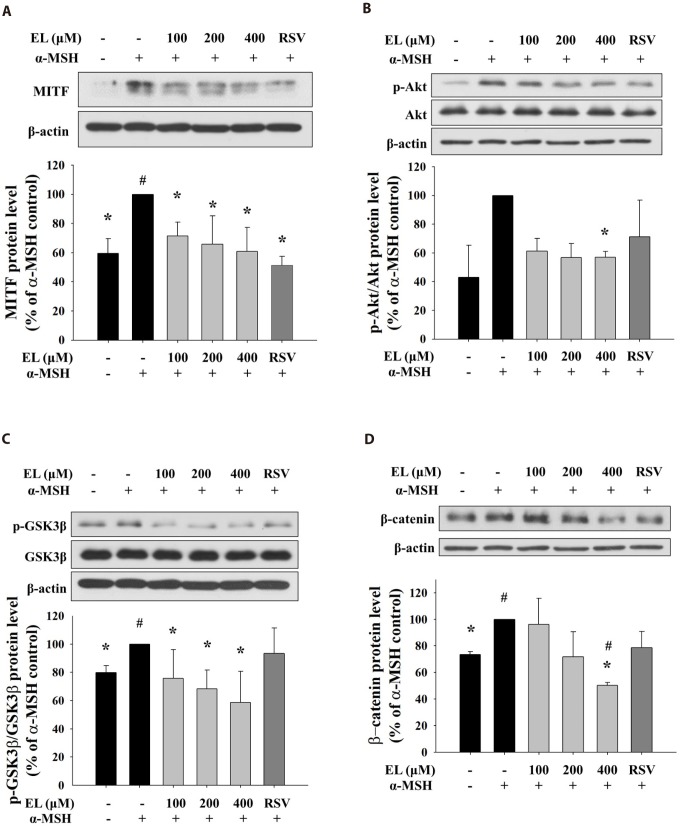
- Ethyl linoleate is an unsaturated fatty acid used in many cosmetics for its various attributes, such as antibacterial and anti-inflammatory properties and clinically proven to be an effective anti-acne agent. In this study, we investigated the effect of ethyl linoleate on the melanogenesis and the mechanism underlying its action on melanogenesis in B16F10 murine melanoma cells. Our results revealed that ethyl linoleate significantly inhibited melanin content and intracellular tyrosinase activity in α-MSH-induced B16F10 cells, but it did not directly inhibit activity of mushroom tyrosinase. Ethyl linoleate inhibited the expression of microphthalmia-associated transcription factor (MITF), tyrosinase, and tyrosinase related protein 1 (TRP1) in governing melanin pigment synthesis. We observed that ethyl linoleate inhibited phosphorylation of Akt and glycogen synthase kinase 3β (GSK3β) and reduced the level of β-catenin, suggesting that ethyl linoleate inhibits melanogenesis through Akt/GSK3β/β-catenin signal pathway. Therefore, we propose that ethyl linoleate may be useful as a safe whitening agent in cosmetic and a potential therapeutic agent for reducing skin hyperpigmentation in clinics.
Keyword
MeSH Terms
-
Agaricales
Glycogen Synthase Kinases
Hyperpigmentation
Linoleic Acid*
Melanins
Melanoma
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Phosphorylation
Signal Transduction*
Skin
Glycogen Synthase Kinases
Linoleic Acid
Melanins
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Figure
Reference
-
1. Clarys P, Alewaeters K, Lambrecht R, Barel AO. Skin color measurements: comparison between three instruments: the Chromameter®, the DermaSpectrometer® and the Mexameter®. Skin Res Technol. 2000; 6:230–238. PMID: 11428962.
Article2. Sturm RA. Skin colour and skin cancer - MC1R, the genetic link. Melanoma Res. 2002; 12:405–416. PMID: 12394181.3. Shoag J, Haq R, Zhang M, Liu L, Rowe GC, Jiang A, Koulisis N, Farrel C, Amos CI, Wei Q, Lee JE, Zhang J, Kupper TS, Qureshi AA, Cui R, Han J, Fisher DE, Arany Z. PGC-1 coactivators regulate MITF and the tanning response. Mol Cell. 2013; 49:145–157. PMID: 23201126.
Article4. Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007; 128:853–864. PMID: 17350573.
Article5. Hsiao JJ, Fisher DE. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch Biochem Biophys. 2014; 563:28–34. PMID: 25111671.
Article6. Speeckaert R, Van Gele M, Speeckaert MM, Lambert J, van Geel N. The biology of hyperpigmentation syndromes. Pigment Cell Melanoma Res. 2014; 27:512–524. PMID: 24612852.
Article7. Chung KW, Jeong HO, Jang EJ, Choi YJ, Kim DH, Kim SR, Lee KJ, Lee HJ, Chun P, Byun Y, Moon HR, Chung HY. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim Biophys Acta. 2013; 1830:4752–4761. PMID: 23769841.
Article8. Hearing VJ. Determination of melanin synthetic pathways. J Invest Dermatol. 2011; 131:E8–E11. PMID: 22094404.
Article9. Jacquemin P, Lannoy VJ, O'Sullivan J, Read A, Lemaigre FP, Rousseau GG. The transcription factor onecut-2 controls the microphthalmia-associated transcription factor gene. Biochem Biophys Res Commun. 2001; 285:1200–1205. PMID: 11478782.
Article10. Saito H, Yasumoto K, Takeda K, Takahashi K, Fukuzaki A, Orikasa S, Shibahara S. Melanocyte-specific microphthalmia-associated transcription factor isoform activates its own gene promoter through physical interaction with lymphoid-enhancing factor 1. J Biol Chem. 2002; 277:28787–28794. PMID: 12048204.
Article11. Bondurand N, Pingault V, Goerich DE, Lemort N, Sock E, Le Caignec C, Wegner M, Goossens M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum Mol Genet. 2000; 9:1907–1917. PMID: 10942418.
Article12. Price ER, Horstmann MA, Wells AG, Weilbaecher KN, Takemoto CM, Landis MW, Fisher DE. α-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. J Biol Chem. 1998; 273:33042–33047. PMID: 9830058.
Article13. Zhu PY, Yin WH, Wang MR, Dang YY, Ye XY. Andrographolide suppresses melanin synthesis through Akt/GSK3β/β-catenin signal pathway. J Dermatol Sci. 2015; 79:74–83. PMID: 25869056.
Article14. Hwang I, Park JH, Park HS, Choi KA, Seol KC, Oh SI, Kang S, Hong S. Neural stem cells inhibit melanin production by activation of Wnt inhibitors. J Dermatol Sci. 2013; 72:274–283. PMID: 24016750.
Article15. Bellei B, Pitisci A, Catricala C, Larue L, Picardo M. Wnt/β-catenin signaling is stimulated by α-melanocyte-stimulating hormone in melanoma and melanocyte cells: implication in cell differentiation. Pigment Cell Melanoma Res. 2011; 24:309–325. PMID: 21040502.
Article16. Nakagawa M, Kawai K, Kawai K. Contact allergy to kojic acid in skin care products. Contact Dermatitis. 1995; 32:9–13. PMID: 7720390.
Article17. Takizawa T, Imai T, Onose J, Ueda M, Tamura T, Mitsumori K, Izumi K, Hirose M. Enhancement of hepatocarcinogenesis by kojic acid in rat two-stage models after initiation with N-bis(2-hydroxypropyl) nitrosamine or N-diethylnitrosamine. Toxicol Sci. 2004; 81:43–49. PMID: 15201437.18. Cheng SL, Liu RH, Sheu JN, Chen ST, Sinchaikul S, Tsay GJ. Toxicogenomics of A375 human malignant melanoma cells treated with arbutin. J Biomed Sci. 2007; 14:87–105. PMID: 17103032.
Article19. Bolognia JL, Sodi SA, Osber MP, Pawelek JM. Enhancement of the depigmenting effect of hydroquinone by cystamine and buthionine sulfoximine. Br J Dermatol. 1995; 133:349–357. PMID: 8546987.
Article20. Makino ET, Mehta RC, Banga A, Jain P, Sigler ML, Sonti S. Evaluation of a hydroquinone-free skin brightening product using in vitro inhibition of melanogenesis and clinical reduction of ultraviolet-induced hyperpigmentation. J Drugs Dermatol. 2013; 12:s16–s20. PMID: 23545928.21. Baynes RE, Hodgson E. Absorption and distribution of toxicants. In : Hodgson E, editor. A textbook of modern toxicology. 3rd ed. New Jersey: John Wiley & Sons;2004. p. 75–110.22. Lee MH, Kim HJ, Ha DJ, Paik JH, Kim HY. Therapeutic effect of topical application of linoleic acid and lincomycin in combination with betamethasone valerate in melasma patients. J Korean Med Sci. 2002; 17:518–523. PMID: 12172049.
Article23. Thirion L, Piérard-Franchimont C, Piérard GE. Whitening effect of a dermocosmetic formulation: a randomized double-blind controlled study on melasma. Int J Cosmet Sci. 2006; 28:263–267. PMID: 18489266.
Article24. Morganti P, Ruocco E, Wolf R, Ruocco V. Percutaneous absorption and delivery systems. Clin Dermatol. 2001; 19:489–501. PMID: 11535394.25. Ando H, Ryu A, Hashimoto A, Oka M, Ichihashi M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch Dermatol Res. 1998; 290:375–381. PMID: 9749992.26. Ando H, Watabe H, Valencia JC, Yasumoto K, Furumura M, Funasaka Y, Oka M, Ichihashi M, Hearing VJ. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: a new aspect of ubiquitin-proteasome function. J Biol Chem. 2004; 279:15427–15433. PMID: 14739285.27. Ando H, Wen ZM, Kim HY, Valencia JC, Costin GE, Watabe H, Yasumoto K, Niki Y, Kondoh H, Ichihashi M, Hearing VJ. Intracellular composition of fatty acid affects the processing and function of tyrosinase through the ubiquitin-proteasome pathway. Biochem J. 2006; 394:43–50. PMID: 16232122.
Article28. Park SY, Seetharaman R, Ko MJ, Kim DY, Kim TH, Yoon MK, Kwak JH, Lee SJ, Bae YS, Choi YW. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int Immunopharmacol. 2014; 19:253–261. PMID: 24508058.
Article29. Jelenko C, Wheeler ML, Anderson AP, Callaway BD, McKinley JC. Studies in burns: XIV, Heling in burn wounds treated with Ethyl Linoleate alone or in combination with selected topical antibacterial agents. Ann Surg. 1975; 182:562–566. PMID: 1190861.30. Charakida A, Charakida M, Chu AC. Double-blind, randomized, placebo-controlled study of a lotion containing triethyl citrate and ethyl linoleate in the treatment of acne vulgaris. Br J Dermatol. 2007; 157:569–574. PMID: 17635508.
Article31. Huh S, Kim YS, Jung E, Lim J, Jung KS, Kim MO, Lee J, Park D. Melanogenesis inhibitory effect of fatty acid alkyl esters isolated from Oxalis triangularis. Biol Pharm Bull. 2010; 33:1242–1245. PMID: 20606321.
Article32. Hosoi J, Abe E, Suda T, Kuroki T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1α,25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985; 45:1474–1478. PMID: 2983883.33. Newton RA, Cook AL, Roberts DW, Leonard JH, Sturm RA. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J Invest Dermatol. 2007; 127:2216–2227. PMID: 17460731.
Article34. Takahashi H, Parsons PG. Rapid and reversible inhibition of tyrosinase activity by glucosidase inhibitors in human melanoma cells. J Invest Dermatol. 1992; 98:481–487. PMID: 1532183.
Article35. Song YW, Cho SK. Phytol induces apoptosis and ROS-mediated protective autophagy in human gastric adenocarcinoma AGS cells. Biochem Anal Biochem. 2015; 4:4.
Article36. Lehman AJ, Patterson WI, Davidow B, Hagan EC, Woodard G, Laug EP, Frawley JP, Fitzhugh OG, Bourke AR, Draize JH, Nelson AA, Vos BJ. Procedures for the appraisal of the toxicity of chemicals in foods, drugs and cosmetics. Food Drug Cosmet Law J. 1955; 10:679–748.37. Tomankova K, Kejlova K, Binder S, Daskova A, Zapletalova J, Bendova H, Kolarova H, Jirova D. In vitro cytotoxicity and phototoxicity study of cosmetics colorants. Toxicol In Vitro. 2011; 25:1242–1250. PMID: 21570462.
Article38. Ando H, Kondoh H, Ichihashi M, Hearing VJ. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol. 2007; 127:751–761. PMID: 17218941.
Article39. Park KC, Huh SY, Choi HR, Kim DS. Biology of melanogenesis and the search for hypopigmenting agents. Dermatologica Sinica. 2010; 28:53–58.
Article40. Oka M, Nagai H, Ando H, Fukunaga M, Matsumura M, Araki K, Ogawa W, Miki T, Sakaue M, Tsukamoto K, Konishi H, Kikkawa U, Ichihashi M. Regulation of melanogenesis through phosphatidylinositol 3-kinase-Akt pathway in human G361 melanoma cells. J Invest Dermatol. 2000; 115:699–703. PMID: 10998146.
Article41. Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, Fisher DZ, Fisher DE. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000; 14:301–312. PMID: 10673502.
Article42. Mansky KC, Sankar U, Han J, Ostrowski MC. Microphthalmia transcription factor is a target of the p38 MAPK pathway in response to receptor activator of NF-κB ligand signaling. J Biol Chem. 2002; 277:11077–11083. PMID: 11792706.
Article43. Bu J, Ma PC, Chen ZQ, Zhou WQ, Fu YJ, Li LJ, Li CR. Inhibition of MITF and tyrosinase by paeonol-stimulated JNK/SAPK to reduction of phosphorylated CREB. Am J Chin Med. 2008; 36:245–263. PMID: 18457359.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sageretia thea fruit extracts rich in methyl linoleate and methyl linolenate downregulate melanogenesis via the Akt/GSK3β signaling pathway
- β-carotene Inhibits Expression of c-Myc and Cyclin E in Helicobacter pylori-infected Gastric Epithelial Cells
- Baicalein Inhibits αα-Melanocyte-stimulating Hormonestimulated Melanogenesis via p38 Mitogen-activated Protein Kinase Pathway in B16F10 Mouse Melanoma Cells
- Effects of microRNA-135a on the epithelial–mesenchymal transition, migration and invasion of bladder cancer cells by targeting GSK3β through the Wnt/β-catenin signaling pathway
- Involvement of Transglutaminase-2 in alpha-MSH-Induced Melanogenesis in SK-MEL-2 Human Melanoma Cells