J Vet Sci.
2018 Nov;19(6):750-758. 10.4142/jvs.2018.19.6.750.
Neonatal influenza virus infection affects myelination in influenza-recovered mouse brain
- Affiliations
-
- 1Department of Veterinary Medicine, College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea. ssnahm@konkuk.ac.kr
- KMID: 2427023
- DOI: http://doi.org/10.4142/jvs.2018.19.6.750
Abstract
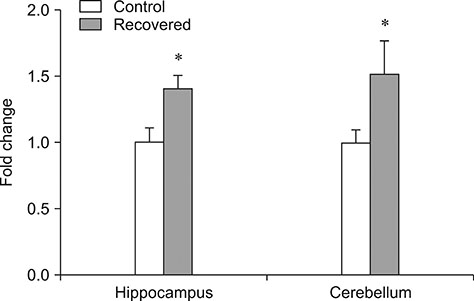
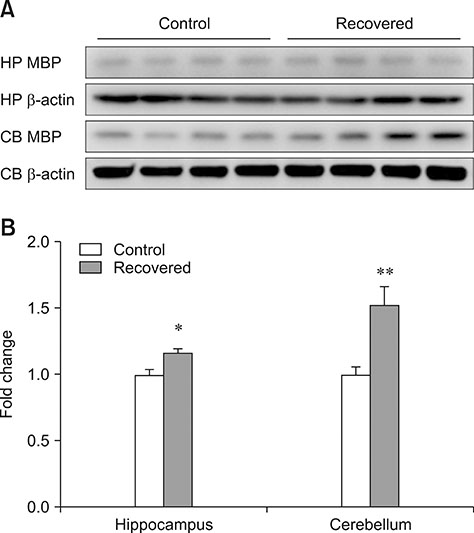
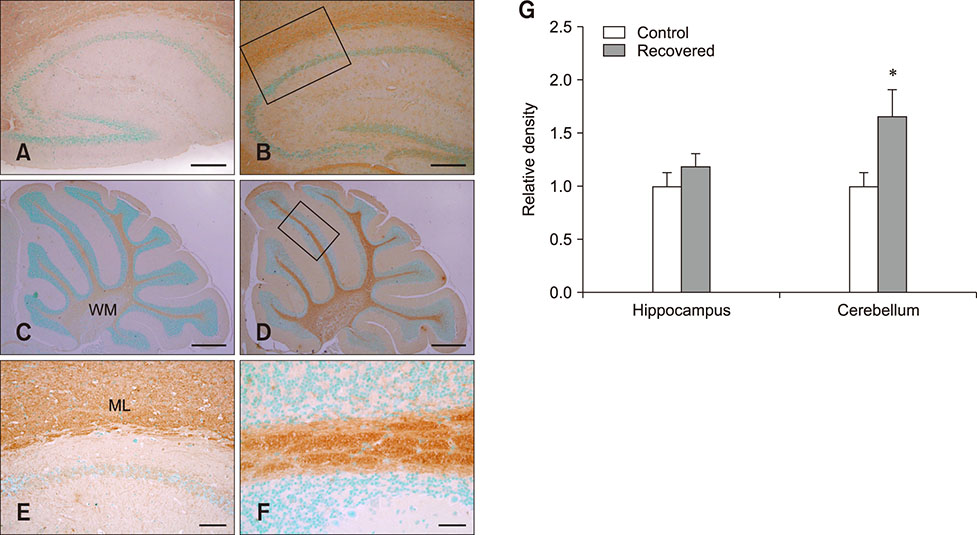
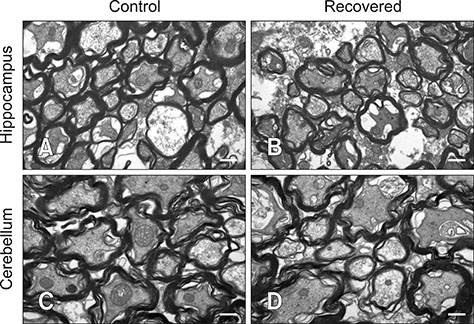
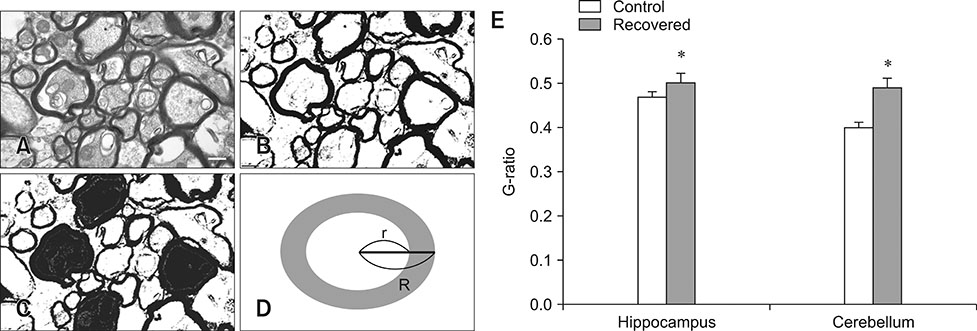
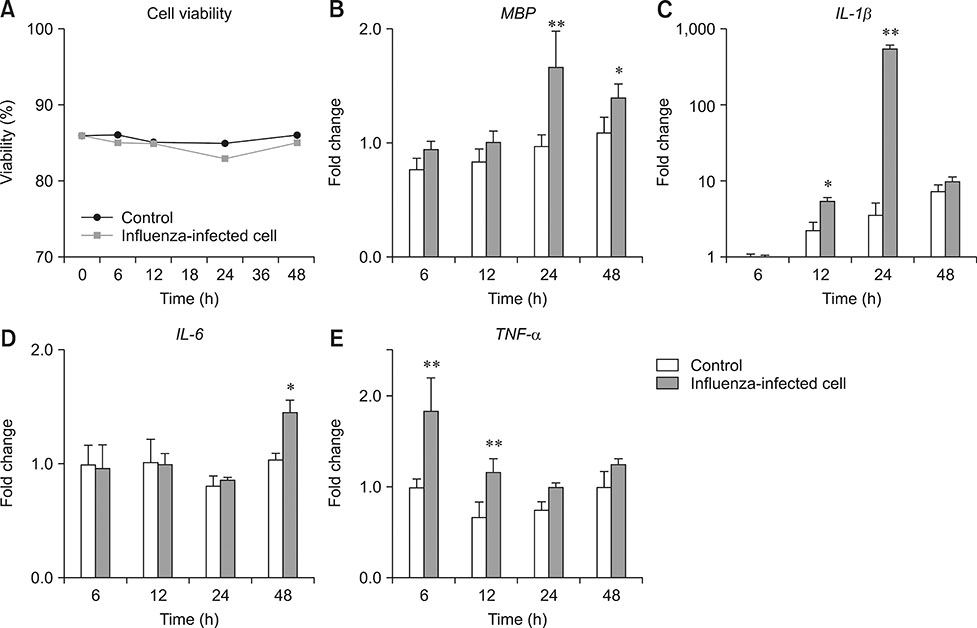
- Influenza virus infection is a zoonosis that has great socioeconomic effects worldwide. Influenza infection induces respiratory symptoms, while the influenza virus can infect brain and leave central nervous system sequelae. As children are more vulnerable to infection, they are at risk of long-term neurological effects once their brains are infected. We previously demonstrated that functional changes in hippocampal neurons were observed in mice recovered from neonatal influenza infection. In this study, we investigated changes in myelination properties that could affect neural dysfunction. Mice were infected with the influenza virus on postnatal day 5. Tissues were harvested from recovered mice 21-days post-infection. The expression levels for myelin basic protein (MBP) were determined, and immunohistochemical staining and transmission electron microscopy were performed. Real-time polymerase chain reaction and Western blot analyses showed that mRNA and protein expressions increased in the hippocampus and cerebellum of recovered mice. Increased MBP-staining signal was observed in the recovered mouse brain. By calculating the relative thickness of myelin sheath in relation to nerve fiber diameter (G-ratio) from electron photomicrographs, an increased G-ratio was observed in both the hippocampus and cerebellum of recovered mice. Influenza infection in oligodendrocyte-enriched primary brain cell cultures showed that proinflammatory cytokines may induce MBP upregulation. These results suggested that increased MBP expression could be a compensatory change related to hypomyelination, which may underlie neural dysfunction in recovered mice. In summary, the present results demonstrate that influenza infection during the neonatal period affects myelination and further induces functional changes in influenza-recovered mouse brain.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Brain*
Cell Culture Techniques
Central Nervous System
Cerebellum
Child
Cytokines
Hippocampus
Humans
Influenza, Human*
Mice*
Microscopy, Electron, Transmission
Myelin Basic Protein
Myelin Sheath*
Nerve Fibers
Neurons
Oligodendroglia
Orthomyxoviridae*
Real-Time Polymerase Chain Reaction
RNA, Messenger
Up-Regulation
Cytokines
Myelin Basic Protein
RNA, Messenger
Figure
Reference
-
1. Baltagi SA, Shoykhet M, Felmet K, Kochanek PM, Bell MJ. Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Pediatr Crit Care Med. 2010; 11:179–184.
Article2. Boullerne AI. The history of myelin. Exp Neurol. 2016; 283:431–445.
Article3. Campbell JSW, Leppert IR, Narayanan S, Boudreau M, Duval T, Cohen-Adad J, Pike GB, Stikov N. Promise and pitfalls of g-ratio estimation with MRI. Neuroimage. 2018; 182:80–96.
Article4. Chambers JS, Perrone-Bizzozero NI. Altered myelination of the hippocampal formation in subjects with schizophrenia and bipolar disorder. Neurochem Res. 2004; 29:2293–2302.
Article5. Chaves AJ, Vergara-Alert J, Busquets N, Valle R, Rivas R, Ramis A, Darji A, Majó N. Neuroinvasion of the highly pathogenic influenza virus H7N1 is caused by disruption of the blood brain barrier in an avian model. PLoS One. 2014; 9:e115138.
Article6. Fatemi SH, Folsom TD, Reutiman TJ, Abu-Odeh D, Mori S, Huang H, Oishi K. Abnormal expression of myelination genes and alterations in white matter fractional anisotropy following prenatal viral influenza infection at E16 in mice. Schizophr Res. 2009; 112:46–53.
Article7. Fatemi SH, Folsom TD, Reutiman TJ, Huang H, Oishi K, Mori S. Prenatal viral infection of mice at E16 causes changes in gene expression in hippocampi of the offspring. Eur Neuropsychopharmacol. 2009; 19:648–653.
Article8. Goenka A, Michael BD, Ledger E, Hart IJ, Absoud M, Chow G, Lilleker J, Lunn M, McKee D, Peake D, Pysden K, Roberts M, Carrol ED, Lim M, Avula S, Solomon T, Kneen R. Neurological manifestations of influenza infection in children and adults: results of a National British Surveillance Study. Clin Infect Dis. 2014; 58:775–784.
Article9. Ishida Y, Kawashima H, Morichi S, Yamanaka G, Okumura A, Nakagawa S, Morishima T. Brain magnetic resonance imaging in acute phase of pandemic influenza A (H1N1) 2009-associated encephalopathy in children. Neuropediatrics. 2015; 46:20–25.
Article10. Jahn O, Tenzer S, Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol. 2009; 40:55–72.
Article11. Jang H, Boltz D, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster R, Smeyne RJ. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A. 2009; 106:14063–14068.
Article12. Kim M, Yu JE, Lee JH, Chang BJ, Song CS, Lee B, Paik DJ, Nahm SS. Comparative analyses of influenza virus receptor distribution in the human and mouse brains. J Chem Neuroanat. 2013; 52:49–57.
Article13. Kıray H, Lindsay SL, Hosseinzadeh S, Barnett SC. The multifaceted role of astrocytes in regulating myelination. Exp Neurol. 2016; 283:541–549.
Article14. Kneeland RE, Fatemi SH. Viral infection, inflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013; 42:35–48.
Article15. Kristensson K. Avian influenza and the brain: comments on the occasion of resurrection of the Spanish flu virus. Brain Res Bull. 2006; 68:406–413.
Article16. Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004; 89:1092–1100.
Article17. Matsuda K, Park CH, Sunden Y, Kimura T, Ochiai K, Kida H, Umemura T. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza A virus in mice. Vet Pathol. 2004; 41:101–107.
Article18. McMurran CE, Jones CA, Fitzgerald DC, Franklin RJ. CNS remyelination and the innate immune system. Front Cell Dev Biol. 2016; 4:38.
Article19. Michel K, Zhao T, Karl M, Lewis K, Fyffe-Maricich SL. Translational control of myelin basic protein expression by ERK2 MAP kinase regulates timely remyelination in the adult brain. J Neurosci. 2015; 35:7850–7865.
Article20. Mori I, Kimura Y. Apoptotic neurodegeneration induced by influenza A virus infection in the mouse brain. Microbes Infect. 2000; 2:1329–1334.
Article21. Park CH, Ishinaka M, Takada A, Kida H, Kimura T, Ochiai K, Umemura T. The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch Virol. 2002; 147:1425–1436.
Article22. Park H, Yu JE, Kim S, Nahm SS, Chung C. Decreased Na+ influx lowers hippocampal neuronal excitability in a mouse model of neonatal influenza infection. Sci Rep. 2015; 5:13440.
Article23. Patel JR, Williams JL, Muccigrosso MM, Liu L, Sun T, Rubin JB, Klein RS. Astrocyte TNFR2 is required for CXCL12-mediated regulation of oligodendrocyte progenitor proliferation and differentiation within the adult CNS. Acta Neuropathol. 2012; 124:847–860.
Article24. Purger D, Gibson EM, Monje M. Myelin plasticity in the central nervous system. Neuropharmacology. 2016; 110:563–573.
Article25. Rawji KS, Mishra MK, Yong VW. Regenerative capacity of macrophages for remyelination. Front Cell Dev Biol. 2016; 20:47.
Article26. Takahashi M, Yamada T, Nakajima S, Nakajima K, Yamamoto T, Okada H. The substantia nigra is a major target for neurovirulent influenza A virus. J Exp Med. 1995; 181:2161–2169.
Article27. Walker CK, Roche JK, Sinha V, Roberts RC. Substantia nigra ultrastructural pathology in schizophrenia. Schizophr Res. 2018; 197:209–218.
Article28. Wang S, Le TQ, Kurihara N, Chida J, Cisse Y, Yano M, Kido H. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J Infect Dis. 2010; 202:991–1001.
Article29. Wilking AN, Elliott E, Garcia MN, Murray KO, Munoz FM. Central nervous system manifestations in pediatric patients with influenza A H1N1 infection during the 2009 pandemic. Pediatr Neurol. 2014; 51:370–376.
Article30. Wong JY, Kelly H, Ip DK, Wu JT, Leung GM, Cowling BJ. Case fatality risk of influenza A (H1N1pdm09): a systematic review. Epidemiology. 2013; 24:830–841.31. Wu S, Wei Z, Greene CM, Yang P, Su J, Song Y, Iuliano AD, Wang Q. Mortality burden from seasonal influenza and 2009 H1N1 pandemic influenza in Beijing, China, 2007–2013. Influenza Other Respir Viruses. 2018; 12:88–97.
Article32. Yamada M, Bingham J, Payne J, Rookes J, Lowther S, Haining J, Robinson R, Johnson D, Middleton D. Multiple routes of invasion of wild-type Clade 1 highly pathogenic avian influenza H5N1 virus into the central nervous system (CNS) after intranasal exposure in ferrets. Acta Neuropathol. 2012; 124:505–516.
Article33. Yoganathan S, Sudhakar SV, James EJ, Thomas MM. Acute necrotising encephalopathy in a child with H1N1 influenza infection: a clinicoradiological diagnosis and follow-up. BMJ Case Rep. 2016; 2016:bcr2015213429.
Article34. Yu JE, Kim M, Lee J, Chang BJ, Song CS, Nahm SS. Neonatal influenza infection causes pathological changes in the mouse brain. Vet Res. 2014; 45:63.
Article35. Zeng H, Quinet S, Huang W, Gan Y, Han C, He Y, Wang Y. Clinical and MRI features of neurological complications after influenza A (H1N1) infection in critically ill children. Pediatr Radiol. 2013; 43:1182–1189.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention and Treatment of Influenza
- Benign Acute Childhood Myositis Associated with Influenza B Virus
- Influenza A Outbreak in a Neonatal Intensive Care Unit During the 2011-2012 Influenza Season in Korea
- Atypical Kawasaki Disease Presenting as Acute Kidney Injury in a Patient with Influenza B Virus Infection
- The significance of avian influenza virus mouse-adaptation and its application in characterizing the efficacy of new vaccines and therapeutic agents