Korean J Radiol.
2018 Feb;19(1):153-157. 10.3348/kjr.2018.19.1.153.
In-vivo Visualization of Iron Oxide Enhancement in Focal Pulmonary Inflammatory Lesions Using a Three-Dimensional Radial Gradient-Echo-Based Ultrashort Echo Time Sequence: A Preliminary Study
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul 03080, Korea.
- 2Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul 03080, Korea.
- 3Department of Biomedical Engineering, Center for Neuroscience Imaging Research, Sungkyunkwan University, Suwon 16419, Korea. jyparu@skku.edu
- KMID: 2425121
- DOI: http://doi.org/10.3348/kjr.2018.19.1.153
Abstract
OBJECTIVE
To preliminarily evaluate technical feasibility of a dual-echo ultrashort echo time (UTE) subtraction MR imaging by using concurrent dephasing and excitation (CODE) sequence for visualization of iron-oxide enhancement in focal inflammatory pulmonary lesions.
MATERIALS AND METHODS
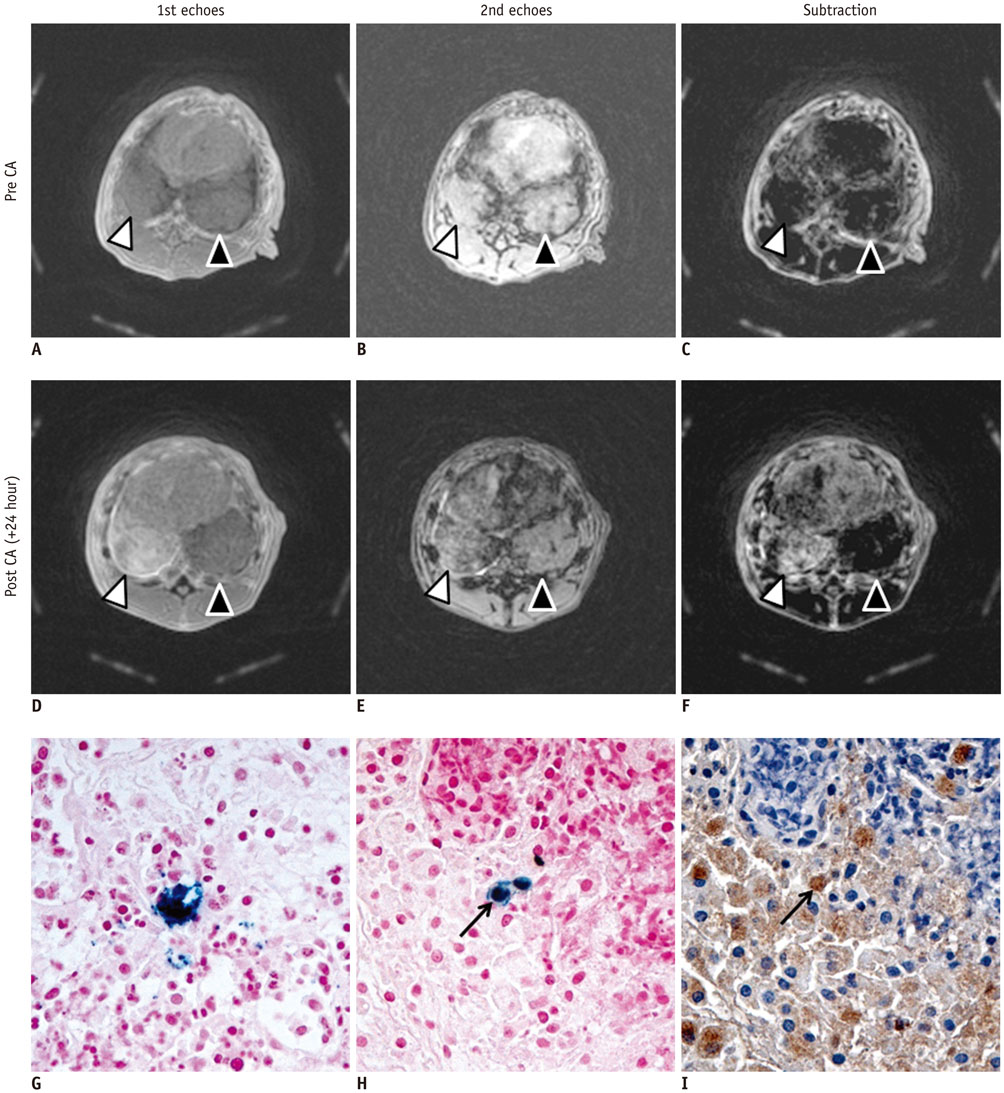
A UTE pulmonary MR imaging before and after the injection of clinically usable superparamagnetic iron-oxide nanoparticles, ferumoxytol, was conducted using CODE sequence with dual echo times of 0.14 ms for the first echo and 4.15 ms for the second echo on 3T scanner in two rabbits concurrently having granulomatous lung disease and lung cancer in separate lobes. A mean ratio of standardized signal intensity (SI) was calculated for comparison of granulomatous lesion and cancer at first echo, second echo, and subtracted images. Lesions were pathologically evaluated with Prussian blue and immunohistochemistry staining.
RESULTS
Post-contrast subtracted CODE images visualized exclusive enhancement of iron oxide in granulomatous disease, but not in the cancer (mean ratio of SI, 2.15 ± 0.68 for granulomatous lesion versus 1.00 ± 0.07 for cancer; p value = 0.002). Prussian blue and corresponding anti-rabbit macrophage IgG-staining suggested an intracellular uptake of iron-oxide nanoparticles in macrophages of granulomatous lesions.
CONCLUSION
Dual-echo UTE subtraction MR imaging using CODE sequence depicts an exclusive positive enhancement of iron-oxide nanoparticle in rabbits in focal granulomatous inflammatory lesions.
MeSH Terms
Figure
Reference
-
1. Girard OM, Du J, Agemy L, Sugahara KN, Kotamraju VR, Ruoslahti E, et al. Optimization of iron oxide nanoparticle detection using ultrashort echo time pulse sequences: comparison of T1, T2*, and synergistic T1- T2* contrast mechanisms. Magn Reson Med. 2011; 65:1649–1660.2. Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: projection reconstruction MR imaging. Radiology. 1991; 179:777–781.
Article3. Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004; 39:56–63.4. Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater. 2014; 13:125–138.
Article5. Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013; 70:1241–1250.
Article6. Park JY, Moeller S, Goerke U, Auerbach E, Chamberlain R, Ellermann J, et al. Short echo-time 3D radial gradient-echo MRI using concurrent dephasing and excitation. Magn Reson Med. 2012; 67:428–436.
Article7. Yoo RE, Choi SH, Cho HR, Jeon BS, Kwon E, Kim EG, et al. Magnetic resonance imaging diagnosis of metastatic lymph nodes in a rabbit model: efficacy of PJY10, a new ultrasmall superparamagnetic iron oxide agent, with monodisperse iron oxide core and multiple-interaction ligands. PLoS One. 2014; 9:e107583.
Article8. Strobel K, Hoerr V, Schmid F, Wachsmuth L, Löffler B, Faber C. Early detection of lung inflammation: exploiting T1-effects of iron oxide particles using UTE MRI. Magn Reson Med. 2012; 68:1924–1931.9. Moore RD, Schoenberg MD. The response of the histiocytes and macrophages in the lungs of rabbits injected with Freund's adjuvant. Br J Exp Pathol. 1964; 45:488–497.10. Vasanawala SS, Nguyen KL, Hope MD, Bridges MD, Hope TA, Reeder SB, et al. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med. 2016; 75:2107–2111.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multi-slice Multi-echo Pulsed-gradient Spin-echo (MePGSE) Sequence for Diffusion Tensor Imaging MRI: A Preliminary Result
- Characterization of Focal Liver Lesions with Superparamagnetic Iron Oxide-Enhanced MR Imaging: Value of Distributional Phase T1-Weighted Imaging
- Gradient Optimized Gradient-Echo Gradient Moment Nulling Sequences for Flow Compensation of Brain mages
- Transfusional Iron Overload and Choroid Plexus Hemosiderosis in a Pediatric Patient: Brain Magnetic Resonance Imaging Findings
- Ferucarbotran-Enhanced Hepatic MRI at 3T Unit: Quantitative and Qualitative Comparison of Fast Breath-hold Imaging Sequences