Yonsei Med J.
2018 Nov;59(9):1131-1137. 10.3349/ymj.2018.59.9.1131.
Decreased Expression of TRPV4 Channels in HEI-OC1 Cells Induced by High Glucose Is Associated with Hearing Impairment
- Affiliations
-
- 1Department of Endocrinology and Metabolism Disease, Xijing Hospital, Forth Military Medical University, Xi'an, China.
- 2Department of Otorhinolaryngology Head and Neck Surgery, Xijing Hospital, Forth Military Medical University, Xi'an, China. yinglinres@163.com, djzhaxian@21cn.com
- KMID: 2422499
- DOI: http://doi.org/10.3349/ymj.2018.59.9.1131
Abstract
- PURPOSE
Previous reports have shown that hyperglycemia-induced inhibition of transient receptor potential vanilloid sub type 4 (TRPV4), a transient receptor potential ion channel, affects the severity of hearing impairment (HI). In this study, we explored the role of TRPV4 in HI using HEI-OC1 cells exposed to high glucose (HG).
MATERIALS AND METHODS
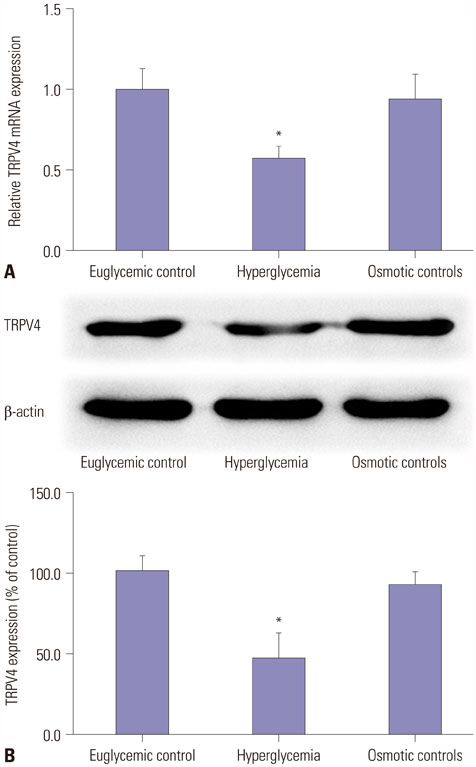
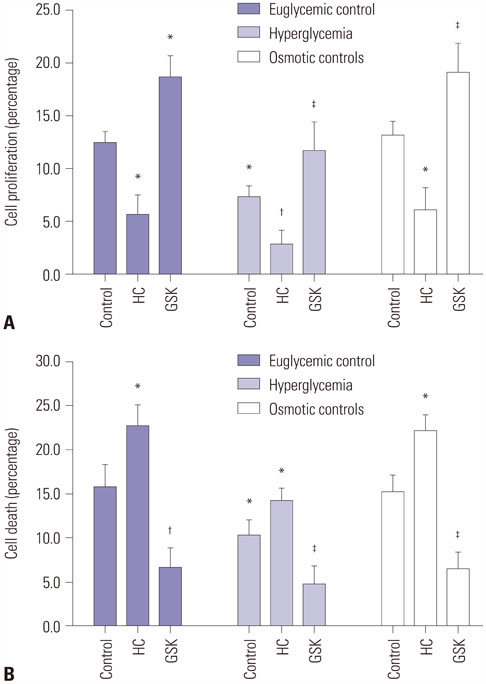
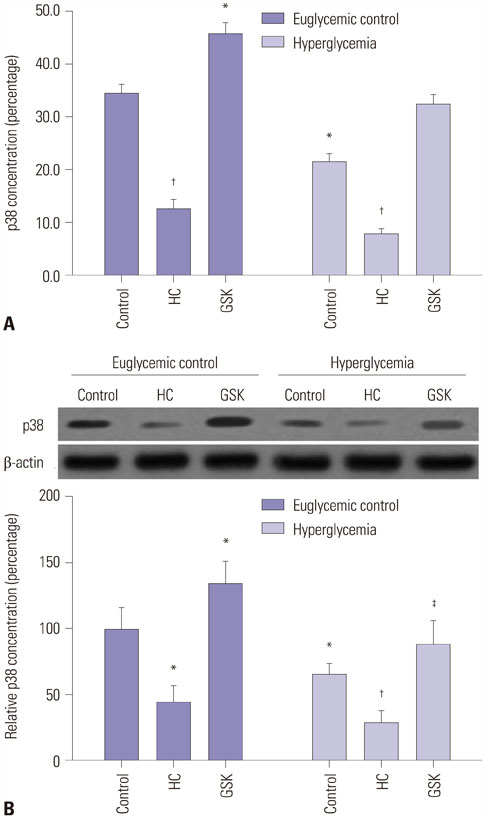
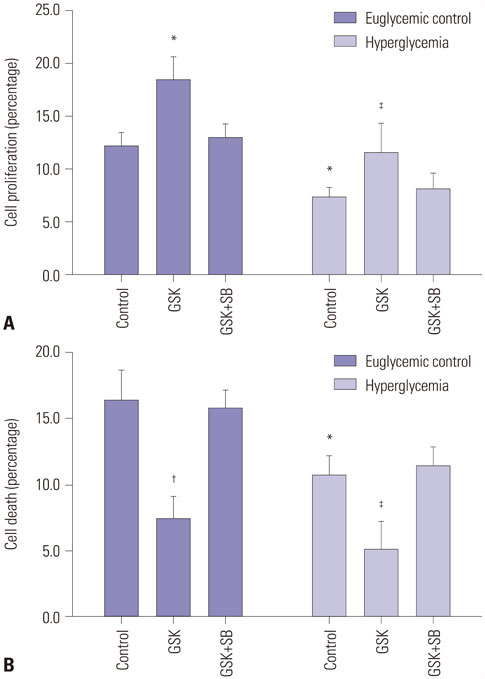
HEI-OC1 cells were cultured in a HG environment (25 mM D-glucose) for 48 hours, and qRT-PCR and Western blotting were used to analyze the expression of TRPV4 at the mRNA and protein level. TRPV4 agonist (GSK1016790A) or antagonist (HC-067047) in cultured HEI-OC1 cells was used to obtain abnormal TRPV4 expression. Functional TRPV4 activity was assessed in cultured HEI-OC1 cells using the MTT assay and a cell death detection ELISA.
RESULTS
TRPV4 agonists exerted protective effects against HG-induced HI, as evidenced by increased MTT levels and inhibition of apoptosis in HEI-OC1 cells. TRPV4 overexpression significantly increased protein levels of phosphorylated p38 mitogen-activated protein kinase (p-p38 MAPK), while TRPV4 antagonists had the opposite effect. Our results indicated that TRPV4 is a hyperglycemia-related factor that can inhibit cell proliferation and promote cell apoptosis by activating the MAPK signaling pathway in HEI-OC1 cells.
CONCLUSION
Our results show that the overexpression of TRPV4 can attenuate cell death in HEI-OC1 cells exposed to HG.
Keyword
MeSH Terms
Figure
Reference
-
1. Genther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. 2013; 309:2322–2324.
Article2. Chia EM, Wang JJ, Rochtchina E, Cumming RR, Newall P, Mitchell P. Hearing impairment and health-related quality of life: the Blue Mountains Hearing Study. Ear Hear. 2007; 28:187–195.
Article3. Gopinath B, Wang JJ, Schneider J, Burlutsky G, Snowdon J, Mc-Mahon CM, et al. Depressive symptoms in older adults with hearing impairments: the Blue Mountains Study. J Am Geriatr Soc. 2009; 57:1306–1308.
Article4. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006; 3:e442.
Article5. Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care: scientific review. JAMA. 2003; 289:1976–1985.
Article6. Hong JW, Jeon JH, Ku CR, Noh JH, Yoo HJ, Kim DJ. The prevalence and factors associated with hearing impairment in the Korean adults: the 2010–2012 Korea National Health and Nutrition Examination Survey (observational study). Medicine (Baltimore). 2015; 94:e611.7. Hirose K. Hearing loss and diabetes: you might not know what you're missing. Ann Intern Med. 2008; 149:54–55.
Article8. Fukushima H, Cureoglu S, Schachern PA, Paparella MM, Harada T, Oktay MF. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg. 2006; 132:934–938.
Article9. Liberman MC, Liberman LD, Maison SF. Chronic conductive hearing loss leads to cochlear degeneration. PLoS One. 2015; 10:e0142341.
Article10. Xue T, Wei L, Zha DJ, Qiu JH, Chen FQ, Qiao L, et al. miR-29b overexpression induces cochlear hair cell apoptosis through the regulation of SIRT1/PGC-1α signaling: Implications for age-related hearing loss. Int J Mol Med. 2016; 38:1387–1394.
Article11. Xu B, Yang L, Lye RJ, Hinton BT. p-MAPK1/3 and DUSP6 regulate epididymal cell proliferation and survival in a region-specific manner in mice. Biol Reprod. 2010; 83:807–817.
Article12. Pan Z, Yang H, Mergler S, Liu H, Tachado SD, Zhang F, et al. Dependence of regulatory volume decrease on transient receptor potential vanilloid 4 (TRPV4) expression in human corneal epithelial cells. Cell Calcium. 2008; 44:374–385.
Article13. Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, et al. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience. 2007; 148:876–892.
Article14. Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci. 2005; 118(Pt 11):2435–2440.
Article15. Lorenzo IM, Liedtke W, Sanderson MJ, Valverde MA. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci U S A. 2008; 105:12611–12616.
Article16. Lee JH, Park C, Kim SJ, Kim HJ, Oh GS, Shen A, et al. Different uptake of gentamicin through TRPV1 and TRPV4 channels determines cochlear hair cell vulnerability. Exp Mol Med. 2013; 45:e12.
Article17. Ma X, Du J, Zhang P, Deng J, Liu J, Lam FF, et al. Functional role of TRPV4-KCa2.3 signaling in vascular endothelial cells in normal and streptozotocin-induced diabetic rats. Hypertension. 2013; 62:134–139.
Article18. Oonk AM, Ekker MS, Huygen PL, Kunst HP, Kremer H, Schelhaas JJ, et al. Intrafamilial variable hearing loss in TRPV4 induced spinal muscular atrophy. Ann Otol Rhinol Laryngol. 2014; 123:859–865.
Article19. Sexton JE, Desmonds T, Quick K, Taylor R, Abramowitz J, Forge A, et al. The contribution of TRPC1, TRPC3, TRPC5 and TRPC6 to touch and hearing. Neurosci Lett. 2016; 610:36–42.
Article20. Monaghan K, McNaughten J, McGahon MK, Kelly C, Kyle D, Yong PH, et al. Hyperglycemia and diabetes downregulate the functional expression of TRPV4 channels in retinal microvascular endothelium. PLoS One. 2015; 10:e0128359.
Article21. Jie P, Hong Z, Tian Y, Li Y, Lin L, Zhou L, et al. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis. 2015; 6:e1775.
Article22. Qu YJ, Zhang X, Fan ZZ, Huai J, Teng YB, Zhang Y, et al. Effect of TRPV4-p38 MAPK pathway on neuropathic pain in rats with chronic compression of the dorsal root ganglion. Biomed Res Int. 2016; 2016:6978923.
Article23. Mihara H, Suzuki N, Boudaka AA, Muhammad JS, Tominaga M, Tabuchi Y, et al. Transient receptor potential vanilloid 4-dependent calcium influx and ATP release in mouse and rat gastric epithelia. World J Gastroenterol. 2016; 22:5512–5519.
Article24. Merrill L, Vizzard MA. Intravesical TRPV4 blockade reduces repeated variate stress-induced bladder dysfunction by increasing bladder capacity and decreasing voiding frequency in male rats. Am J Physiol Regul Integr Comp Physiol. 2014; 307:R471–R480.
Article25. Hong Z, Jie P, Tian Y, Chen T, Chen L, Chen L. Transient receptor potential vanilloid 4-induced modulation of voltage-gated sodium channels in hippocampal neurons. Mol Neurobiol. 2016; 53:759–768.
Article26. Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003; 424:81–84.
Article27. Tabuchi K, Suzuki M, Mizuno A, Hara A. Hearing impairment in TRPV4 knockout mice. Neurosci Lett. 2005; 382:304–308.
Article28. Corey DP, García-Añoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004; 432:723–730.
Article29. Quick K, Zhao J, Eijkelkamp N, Linley JE, Rugiero F, Cox JJ, et al. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol. 2012; 2:120068.
Article30. Zanini D, Göpfert MC. TRPs in hearing. Handb Exp Pharmacol. 2014; 223:899–916.
Article31. Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, et al. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol. 2013; 305:C704–C715.
Article32. Yang X, Bhaskaran A, Scott H, Ardekani S, Xu J, Mohideen U, et al. Rho/ROCK-mediated retinal endothelial stiffening impairs TRPV4 signaling and promotes diabetic retinal inflammation. Invest Ophthalmol Vis Sci. 2016; 57:3220.33. Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007; 7:11.
Article34. Zsombok A, Derbenev AV. TRP channels as therapeutic targets in diabetes and obesity. Pharmaceuticals (Basel). 2016; 9:pii: E50.
Article35. Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003; 23:8596–8607.
Article36. Eshraghi AA, Hoosien G, Ramsay S, Dinh CT, Bas E, Balkany TJ, et al. Inhibition of the JNK signal cascade conserves hearing against electrode insertion trauma-induced loss. Cochlear Implants Int. 2010; 11:Suppl 1. 104–109.
Article37. Van De Water TR, Lallemend F, Eshraghi AA, Ahsan S, He J, Guzman J, et al. Caspases, the enemy within, and their role in oxidative stress-induced apoptosis of inner ear sensory cells. Otol Neurotol. 2004; 25:627–632.
Article38. Tadros SF, D'Souza M, Zhu X, Frisina RD. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis. 2008; 13:1303–1321.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of TRPV4 Channel in Human White Adipocytes Metabolic Activity
- Dexamethasone Inhibits Interleukin-1beta-Induced Matrix Metalloproteinase-9 Expression in Cochlear Cells
- The Effect of IP3 Receptor Inhibition Using 2-APB on Gentamicin Ototoxicity in Cochlear Sensory Cell
- Characterization of Gentamicin-Induced Apoptosis in a Cochlear Cell Model
- Functional Expression of TRPV4 Cation Channels in Human Mast Cell Line (HMC-1)