Endocrinol Metab.
2021 Oct;36(5):997-1006. 10.3803/EnM.2021.1167.
Role of TRPV4 Channel in Human White Adipocytes Metabolic Activity
- Affiliations
-
- 1Faculty of Health Sciences, Technological University of Pereira, La Julita, Pereira, Colombia
- KMID: 2521948
- DOI: http://doi.org/10.3803/EnM.2021.1167
Abstract
- Background
Intracellular calcium (Ca2+) homeostasis plays an essential role in adipocyte metabolism and its alteration is associated with obesity and related disorders. Transient receptor potential vanilloid 4 (TRPV4) channels are an important Ca2+ pathway in adipocytes and their activity is regulated by metabolic mediators such as insulin. In this study, we evaluated the role of TRPV4 channels in metabolic activity and adipokine secretion in human white adipocytes.
Methods
Human white adipocytes were freshly cultured and the effects of the activation and inhibition of TRPV4 channels on lipolysis, glucose uptake, lactate production, and leptin and adiponectin secretion were evaluated.
Results
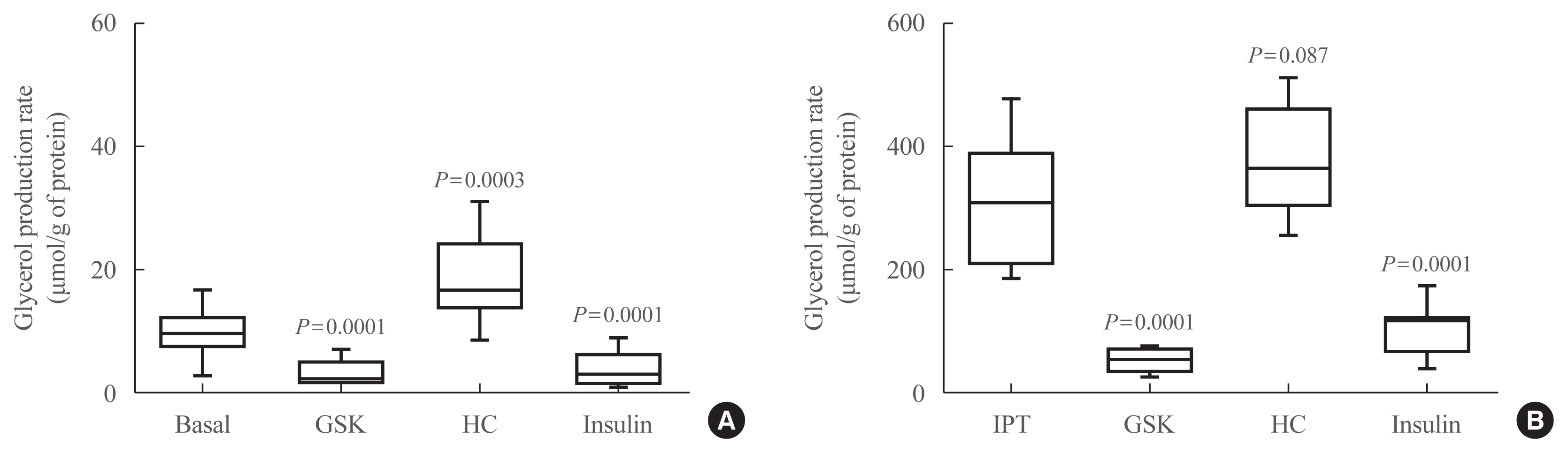
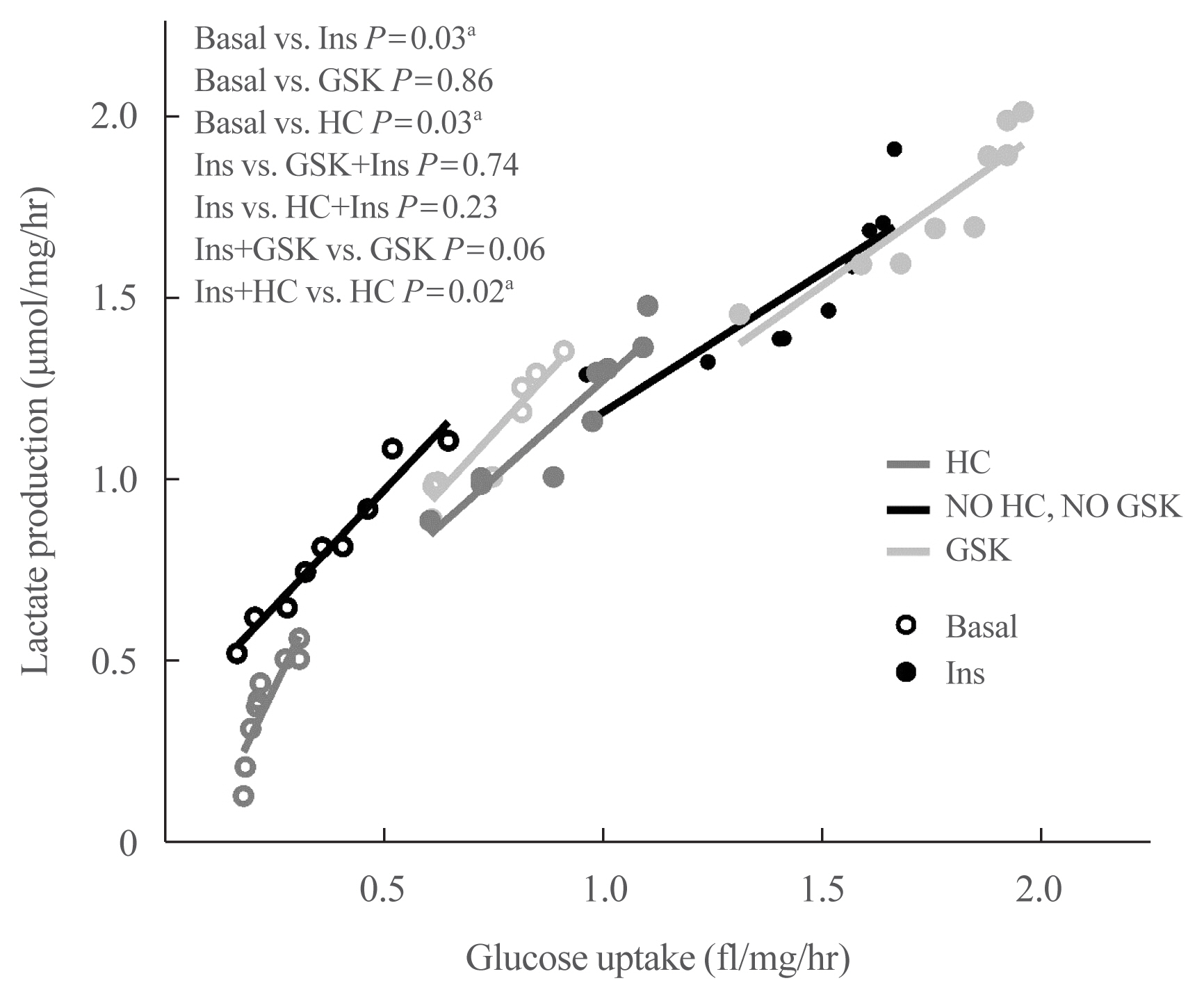
Under basal and isoproterenol-stimulated conditions, TRPV4 activation by GSK1016709A decreased lipolysis whereas HC067047, an antagonist, increased lipolysis. The activation of TRPV4 resulted in increased glucose uptake and lactate production under both basal conditions and insulin-stimulated conditions; in contrast HC067047 decreased both parameters. Leptin production was increased, and adiponectin production was diminished by TRPV4 activation and its inhibition had the opposite effect.
Conclusion
Our results suggested that TRPV4 channels are metabolic mediators involved in proadipogenic processes and glucose metabolism in adipocyte biology. TRPV4 channels could be a potential pharmacological target to treat metabolic disorders.
Keyword
Figure
Reference
-
1. Cifuentes M, Rojas CV. Antilipolytic effect of calcium-sensing receptor in human adipocytes. Mol Cell Biochem. 2008; 319:17–21.
Article2. Cammisotto PG, Bukowiecki LJ. Role of calcium in the secretion of leptin from white adipocytes. Am J Physiol Regul Integr Comp Physiol. 2004; 287:R1380–6.
Article3. Sukumar P, Sedo A, Li J, Wilson LA, O’Regan D, Lippiat JD, et al. Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ Res. 2012; 111:191–200.
Article4. Goudarzi F, Mohammadalipour A, Khodadadi I, Karimi S, Mostoli R, Bahabadi M, et al. The role of calcium in differentiation of human adipose-derived stem cells to adipocytes. Mol Biotechnol. 2018; 60:279–89.
Article5. Schlottmann I, Ehrhart-Bornstein M, Wabitsch M, Bornstein SR, Lamounier-Zepter V. Calcium-dependent release of adipocyte fatty acid binding protein from human adipocytes. Int J Obes (Lond). 2014; 38:1221–7.
Article6. Amitani M, Asakawa A, Amitani H, Inui A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. 2013; 7:51.
Article7. Shan T, Zhang P, Jiang Q, Xiong Y, Wang Y, Kuang S. Adipocyte-specific deletion of mTOR inhibits adipose tissue development and causes insulin resistance in mice. Diabetologia. 2016; 59:1995–2004.
Article8. Pandey GK, Vadivel S, Raghavan S, Mohan V, Balasubramanyam M, Gokulakrishnan K. High molecular weight adiponectin reduces glucolipotoxicity-induced inflammation and improves lipid metabolism and insulin sensitivity via APPL1-AMPK-GLUT4 regulation in 3T3-L1 adipocytes. Atherosclerosis. 2019; 288:67–75.
Article9. Zechner R, Madeo F, Kratky D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol. 2017; 18:671–84.
Article10. Qiao L, Kinney B, Schaack J, Shao J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes. 2011; 60:1519–27.
Article11. Kim SJ, Tang T, Abbott M, Viscarra JA, Wang Y, Sul HS. AMPK phosphorylates desnutrin/ATGL and hormone-sensitive lipase to regulate lipolysis and fatty acid oxidation within adipose tissue. Mol Cell Biol. 2016; 36:1961–76.
Article12. Vincent F, Duncton MA. TRPV4 agonists and antagonists. Curr Top Med Chem. 2011; 11:2216–26.
Article13. Hoshi Y, Okabe K, Shibasaki K, Funatsu T, Matsuki N, Ikegaya Y, et al. Ischemic brain injury leads to brain edema via hyperthermia-induced TRPV4 activation. J Neurosci. 2018; 38:5700–9.
Article14. Olivan-Viguera A, Garcia-Otin AL, Lozano-Gerona J, Abarca-Lachen E, Garcia-Malinis AJ, Hamilton KL, et al. Pharmacological activation of TRPV4 produces immediate cell damage and induction of apoptosis in human melanoma cells and HaCaT keratinocytes. PLoS One. 2018; 13:e0190307.
Article15. Sharma S, Goswami R, Merth M, Cohen J, Lei KY, Zhang DX, et al. TRPV4 ion channel is a novel regulator of dermal myofibroblast differentiation. Am J Physiol Cell Physiol. 2017; 312:C562–72.
Article16. Boehmerle W, Huehnchen P, Lee SL, Harms C, Endres M. TRPV4 inhibition prevents paclitaxel-induced neurotoxicity in preclinical models. Exp Neurol. 2018; 306:64–75.
Article17. Wu Q, Lu K, Zhao Z, Wang B, Liu H, Zhang S, et al. Blockade of transient receptor potential vanilloid 4 enhances antioxidation after myocardial ischemia/reperfusion. Oxid Med Cell Longev. 2019; 2019:7283683.
Article18. White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. TRPV4: molecular conductor of a diverse orchestra. Physiol Rev. 2016; 96:911–73.
Article19. Sanchez JC, Rivera RA, Munoz LV. TRPV4 channels in human white adipocytes: electrophysiological characterization and regulation by insulin. J Cell Physiol. 2016; 231:954–63.
Article20. Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012; 151:96–110.
Article21. Kusudo T, Wang Z, Mizuno A, Suzuki M, Yamashita H. TRPV4 deficiency increases skeletal muscle metabolic capacity and resistance against diet-induced obesity. J Appl Physiol (1985). 2012; 112:1223–32.
Article22. Duan DM, Wu S, Hsu LA, Teng MS, Lin JF, Sun YC, et al. Associations between TRPV4 genotypes and body mass index in Taiwanese subjects. Mol Genet Genomics. 2015; 290:1357–65.
Article23. Palhinha L, Liechocki S, Hottz ED, Pereira JA, de Almeida CJ, Moraes-Vieira PM, et al. Leptin induces proadipogenic and proinflammatory signaling in adipocytes. Front Endocrinol (Lausanne). 2019; 10:841.
Article24. Silha JV, Krsek M, Skrha JV, Sucharda P, Nyomba BL, Murphy LJ. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: correlations with insulin resistance. Eur J Endocrinol. 2003; 149:331–5.
Article25. Ramirez-Ponce MP, Mateos JC, Bellido JA. Human adipose cells have voltage-dependent potassium currents. J Membr Biol. 2003; 196:129–34.
Article26. Pereira MJ, Thombare K, Sarsenbayeva A, Kamble PG, Almby K, Lundqvist M, et al. Direct effects of glucagon on glucose uptake and lipolysis in human adipocytes. Mol Cell Endocrinol. 2020; 503:110696.
Article27. Krycer JR, Quek LE, Francis D, Fazakerley DJ, Elkington SD, Diaz-Vegas A, et al. Lactate production is a prioritized feature of adipocyte metabolism. J Biol Chem. 2020; 295:83–98.
Article28. Xue B, Greenberg AG, Kraemer FB, Zemel MB. Mechanism of intracellular calcium ([Ca2+] i) inhibition of lipolysis in human adipocytes. FASEB J. 2001; 15:2527–9.29. Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000; 103:525–35.
Article30. Ahern GP. Transient receptor potential channels and energy homeostasis. Trends Endocrinol Metab. 2013; 24:554–60.
Article31. Yang S, Lu W, Zhao C, Zhai Y, Wei Y, Liu J, et al. Leukemia cells remodel marrow adipocytes via TRPV4-dependent lipolysis. Haematologica. 2020; 105:2572–83.
Article32. Snyder PB, Esselstyn JM, Loughney K, Wolda SL, Florio VA. The role of cyclic nucleotide phosphodiesterases in the regulation of adipocyte lipolysis. J Lipid Res. 2005; 46:494–503.
Article33. Krycer JR, Quek LE, Francis D, Zadoorian A, Weiss FC, Cooke KC, et al. Insulin signaling requires glucose to promote lipid anabolism in adipocytes. J Biol Chem. 2020; 295:13250–66.
Article34. Krycer JR, Yugi K, Hirayama A, Fazakerley DJ, Quek LE, Scalzo R, et al. Dynamic metabolomics reveals that insulin primes the adipocyte for glucose metabolism. Cell Rep. 2017; 21:3536–47.
Article35. Cai TQ, Ren N, Jin L, Cheng K, Kash S, Chen R, et al. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem Biophys Res Commun. 2008; 377:987–91.
Article36. Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, et al. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010; 11:311–9.
Article37. Whitehead JP, Molero JC, Clark S, Martin S, Meneilly G, James DE. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J Biol Chem. 2001; 276:27816–24.
Article38. Pereira MJ, Palming J, Rizell M, Aureliano M, Carvalho E, Svensson MK, et al. Cyclosporine A and tacrolimus reduce the amount of GLUT4 at the cell surface in human adipocytes: increased endocytosis as a potential mechanism for the diabetogenic effects of immunosuppressive agents. J Clin Endocrinol Metab. 2014; 99:E1885–94.
Article39. Fonseca AC, Carvalho E, Eriksson JW, Pereira MJ. Calcineurin is an important factor involved in glucose uptake in human adipocytes. Mol Cell Biochem. 2018; 445:157–68.
Article40. Pereira MJ, Palming J, Rizell M, Aureliano M, Carvalho E, Svensson MK, et al. The immunosuppressive agents rapamycin, cyclosporin A and tacrolimus increase lipolysis, inhibit lipid storage and alter expression of genes involved in lipid metabolism in human adipose tissue. Mol Cell Endocrinol. 2013; 365:260–9.
Article41. Woody S, Stall R, Ramos J, Patel YM. Regulation of myosin light chain kinase during insulin-stimulated glucose uptake in 3T3-L1 adipocytes. PLoS One. 2013; 8:e77248.
Article42. Page AJ, Hatzinikolas G, Vincent AD, Cavuoto P, Wittert GA. The TRPV1 channel regulates glucose metabolism. Am J Physiol Endocrinol Metab. 2019; 317:E667–76.
Article43. Tang W, Fan Y. SIRT6 as a potential target for treating insulin resistance. Life Sci. 2019; 231:116558.
Article44. Levy JR, Gyarmati J, Lesko JM, Adler RA, Stevens W. Dual regulation of leptin secretion: intracellular energy and calcium dependence of regulated pathway. Am J Physiol Endocrinol Metab. 2000; 278:E892–901.
Article45. Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, et al. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol. 2010; 30:851–8.46. Bradley RL, Cheatham B. Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes. 1999; 48:272–8.
Article47. Ozaki KI, Awazu M, Tamiya M, Iwasaki Y, Harada A, Kugisaki S, et al. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am J Physiol Endocrinol Metab. 2016; 310:E643–51.
Article48. Hu W, Ding Y, Li Q, Shi R, He Y. Transient receptor potential vanilloid 4 channels as therapeutic targets in diabetes and diabetes-related complications. J Diabetes Investig. 2020; 11:757–69.
Article49. Goyal N, Skrdla P, Schroyer R, Kumar S, Fernando D, Oughton A, et al. Clinical pharmacokinetics, safety, and tolerability of a novel, first-in-class TRPV4 ion channel inhibitor, GSK2798745, in healthy and heart failure subjects. Am J Cardiovasc Drugs. 2019; 19:335–42.
Article50. Gao P, Li L, Wei X, Wang M, Hong Y, Wu H, et al. Activation of transient receptor potential channel vanilloid 4 by DPP-4 (dipeptidyl peptidase-4) inhibitor vildagliptin protects against diabetic endothelial dysfunction. Hypertension. 2020; 75:150–62.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Emerging Importance of Mitochondria in White Adipocytes: Neither Last nor Least
- Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation
- Decreased Expression of TRPV4 Channels in HEI-OC1 Cells Induced by High Glucose Is Associated with Hearing Impairment
- Channel Function of TRPML1 Prompts Lipolysis in Mature Adipocytes
- Mitochondrial Dysfunction in Adipocytes as a Primary Cause of Adipose Tissue Inflammation