Endocrinol Metab.
2023 Oct;38(5):493-503. 10.3803/EnM.2023.1813.
The Emerging Importance of Mitochondria in White Adipocytes: Neither Last nor Least
- Affiliations
-
- 1CAS Key Laboratory of Molecular Virology and Immunology, The Center for Microbes, Development and Health, Shanghai Institute of Immunity and Infection, Chinese Academy of Sciences, Shanghai, China
- 2Department of Anesthesiology, Critical Care and Pain Medicine, Center for Perioperative Medicine, McGovern Medical School, UT Health Science Center at Houston, Houston, TX, USA
- KMID: 2546980
- DOI: http://doi.org/10.3803/EnM.2023.1813
Abstract
- The growing recognition of mitochondria’s crucial role in the regulation of white adipose tissue remodeling and energy balance underscores its significance. The marked metabolic diversity of mitochondria provides the molecular and cellular foundation for enabling adipose tissue plasticity in response to various metabolic cues. Effective control of mitochondrial function at the cellular level, not only in thermogenic brown and beige adipocytes but also in energy-storing white adipocytes, exerts a profound influence on adipose homeostasis. Furthermore, mitochondria play a pivotal role in intercellular communication within adipose tissue via production of metabolites with signaling properties. A more comprehensive understanding of mitochondrial regulation within white adipocytes will empower the development of targeted and efficacious strategies to enhance adipose function, leading to advancements in overall metabolic health.
Keyword
Figure
Reference
-
1. McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006; 16:R551–60.
Article2. Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014; 505:335–43.
Article3. Picard M, Shirihai OS. Mitochondrial signal transduction. Cell Metab. 2022; 34:1620–53.
Article4. Monzel AS, Enriquez JA, Picard M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat Metab. 2023; 5:546–62.
Article5. Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018; 20:745–54.
Article6. Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018; 19:713–30.
Article7. Garbincius JF, Elrod JW. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 2022; 102:893–992.
Article8. Bock FJ, Tait SW. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020; 21:85–100.
Article9. Tan JX, Finkel T. Mitochondria as intracellular signaling platforms in health and disease. J Cell Biol. 2020; 219:e202002179.
Article10. Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology: adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007; 48:1253–62.
Article11. Morigny P, Boucher J, Arner P, Langin D. Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat Rev Endocrinol. 2021; 17:276–95.
Article12. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014; 156:20–44.
Article13. Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adiposetissue plasticity in health and disease. Cell. 2022; 185:419–46.
Article14. Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, et al. FAT SIGNALS: lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012; 15:279–91.15. Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009; 48:275–97.
Article16. Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015; 36:461–70.
Article17. Funcke JB, Scherer PE. Beyond adiponectin and leptin: adipose tissue-derived mediators of inter-organ communication. J Lipid Res. 2019; 60:1648–84.
Article18. Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016; 17:691–702.
Article19. Shamsi F, Wang CH, Tseng YH. The evolving view of thermogenic adipocytes: ontogeny, niche and function. Nat Rev Endocrinol. 2021; 17:726–44.
Article20. Cohen P, Kajimura S. The cellular and functional complexity of thermogenic fat. Nat Rev Mol Cell Biol. 2021; 22:393–409.
Article21. Heinonen S, Jokinen R, Rissanen A, Pietilainen KH. White adipose tissue mitochondrial metabolism in health and in obesity. Obes Rev. 2020; 21:e12958.
Article22. Zhu Q, An YA, Scherer PE. Mitochondrial regulation and white adipose tissue homeostasis. Trends Cell Biol. 2022; 32:351–64.
Article23. Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015; 125:478–86.
Article24. Aquilano K, Zhou B, Brestoff JR, Lettieri-Barbato D. Multifaceted mitochondrial quality control in brown adipose tissue. Trends Cell Biol. 2023; 33:517–29.
Article25. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009; 360:1509–17.
Article26. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Coldactivated brown adipose tissue in healthy men. N Engl J Med. 2009; 360:1500–8.
Article27. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009; 360:1518–25.
Article28. Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fattyacid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012; 151:400–13.
Article29. Chouchani ET, Kazak L, Spiegelman BM. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 2019; 29:27–37.
Article30. Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015; 163:643–55.
Article31. Sun Y, Rahbani JF, Jedrychowski MP, Riley CL, Vidoni S, Bogoslavski D, et al. Mitochondrial TNAP controls thermogenesis by hydrolysis of phosphocreatine. Nature. 2021; 593:580–5.
Article32. Rahbani JF, Roesler A, Hussain MF, Samborska B, Dykstra CB, Tsai L, et al. Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature. 2021; 590:480–5.
Article33. Lee JH, Park A, Oh KJ, Lee SC, Kim WK, Bae KH. The role of adipose tissue mitochondria: regulation of mitochondrial function for the treatment of metabolic diseases. Int J Mol Sci. 2019; 20:4924.
Article34. Pagliarini DJ, Rutter J. Hallmarks of a new era in mitochondrial biochemistry. Genes Dev. 2013; 27:2615–27.
Article35. Wiedemann N, Pfanner N. Mitochondrial machineries for protein import and assembly. Annu Rev Biochem. 2017; 86:685–714.
Article36. Ernster L, Schatz G. Mitochondria: a historical review. J Cell Biol. 1981; 91(3 Pt 2):227s–55s.
Article37. De Pauw A, Tejerina S, Raes M, Keijer J, Arnould T. Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am J Pathol. 2009; 175:927–39.
Article38. Vance JE. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015; 16:1–18.
Article39. She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007; 6:181–94.
Article40. Wang CH, Wang CC, Huang HC, Wei YH. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 2013; 280:1039–50.
Article41. Sutherland LN, Capozzi LC, Turchinsky NJ, Bell RC, Wright DC. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: potential mechanisms and the relationship to glucose intolerance. Am J Physiol Endocrinol Metab. 2008; 295:E1076–83.
Article42. Koh EH, Kim M, Ranjan KC, Kim HS, Park HS, Oh KS, et al. eNOS plays a major role in adiponectin synthesis in adipocytes. Am J Physiol Endocrinol Metab. 2010; 298:E846–53.
Article43. Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007; 56:2973–81.
Article44. Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol. 2010; 176:1364–76.
Article45. Rajbhandari P, Arneson D, Hart SK, Ahn IS, Diamante G, Santos LC, et al. Single cell analysis reveals immune celladipocyte crosstalk regulating the transcription of thermogenic adipocytes. Elife. 2019; 8:e49501.
Article46. Sarvari AK, Van Hauwaert EL, Markussen LK, Gammelmark E, Marcher AB, Ebbesen MF, et al. Plasticity of epididymal adipose tissue in response to diet-induced obesity at single-nucleus resolution. Cell Metab. 2021; 33:437–53.
Article47. Whytock KL, Sun Y, Divoux A, Yu G, Smith SR, Walsh MJ, et al. Single cell full-length transcriptome of human subcutaneous adipose tissue reveals unique and heterogeneous cell populations. iScience. 2022; 25:104772.
Article48. Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci. 2004; 117(Pt 18):4055–66.
Article49. Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002; 21:4411–9.
Article50. Jukarainen S, Heinonen S, Ramo JT, Rinnankoski-Tuikka R, Rappou E, Tummers M, et al. Obesity is associated with low NAD(+)/SIRT pathway expression in adipose tissue of BMIdiscordant monozygotic twins. J Clin Endocrinol Metab. 2016; 101:275–83.51. Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007; 450:712–6.
Article52. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006; 127:1109–22.
Article53. Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004; 429:771–6.
Article54. Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007; 6:759–67.
Article55. Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007; 6:105–14.
Article56. Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005; 280:13560–7.
Article57. Bhaskaran S, Pharaoh G, Ranjit R, Murphy A, Matsuzaki S, Nair BC, et al. Loss of mitochondrial protease ClpP protects mice from diet-induced obesity and insulin resistance. EMBO Rep. 2018; 19:e45009.
Article58. Moore TM, Cheng L, Wolf DM, Ngo J, Segawa M, Zhu X, et al. Parkin regulates adiposity by coordinating mitophagy with mitochondrial biogenesis in white adipocytes. Nat Commun. 2022; 13:6661.
Article59. An YA, Crewe C, Asterholm IW, Sun K, Chen S, Zhang F, et al. Dysregulation of amyloid precursor protein impairs adipose tissue mitochondrial function and promotes obesity. Nat Metab. 2019; 1:1243–57.
Article60. An YA, Chen S, Deng Y, Wang ZV, Funcke JB, Shah M, et al. The mitochondrial dicarboxylate carrier prevents hepatic lipotoxicity by inhibiting white adipocyte lipolysis. J Hepatol. 2021; 75:387–99.
Article61. Vernochet C, Damilano F, Mourier A, Bezy O, Mori MA, Smyth G, et al. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. FASEB J. 2014; 28:4408–19.
Article62. Kusminski CM, Ghaben AL, Morley TS, Samms RJ, Adams AC, An Y, et al. A novel model of diabetic complications: adipocyte mitochondrial dysfunction triggers massive β-cell hyperplasia. Diabetes. 2020; 69:313–30.
Article63. Ryu MJ, Kim SJ, Kim YK, Choi MJ, Tadi S, Lee MH, et al. Crif1 deficiency reduces adipose OXPHOS capacity and triggers inflammation and insulin resistance in mice. PLoS Genet. 2013; 9:e1003356.
Article64. Wu H, Wang Y, Li W, Chen H, Du L, Liu D, et al. Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome. Autophagy. 2019; 15:1882–98.
Article65. Choi KM, Ryan KK, Yoon JC. Adipose mitochondrial complex I deficiency modulates inflammation and glucose homeostasis in a sex-dependent manner. Endocrinology. 2022; 163:bqac018.
Article66. Villarroya J, Dorado B, Vila MR, Garcia-Arumi E, Domingo P, Giralt M, et al. Thymidine kinase 2 deficiency-induced mitochondrial DNA depletion causes abnormal development of adipose tissues and adipokine levels in mice. PLoS One. 2011; 6:e29691.
Article67. Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012; 18:1539–49.
Article68. Kusminski CM, Park J, Scherer PE. MitoNEET-mediated effects on browning of white adipose tissue. Nat Commun. 2014; 5:3962.
Article69. Blankenhaus B, Braza F, Martins R, Bastos-Amador P, Gonzalez-Garcia I, Carlos AR, et al. Ferritin regulates organismal energy balance and thermogenesis. Mol Metab. 2019; 24:64–79.
Article70. Ling Y, Carayol J, Galusca B, Canto C, Montaurier C, Matone A, et al. Persistent low body weight in humans is associated with higher mitochondrial activity in white adipose tissue. Am J Clin Nutr. 2019; 110:605–16.
Article71. Mustelin L, Pietilainen KH, Rissanen A, Sovijarvi AR, Piirila P, Naukkarinen J, et al. Acquired obesity and poor physical fitness impair expression of genes of mitochondrial oxidative phosphorylation in monozygotic twins discordant for obesity. Am J Physiol Endocrinol Metab. 2008; 295:E148–54.
Article72. Liguori R, Mazzaccara C, Pasanisi F, Buono P, Oriani G, Finelli C, et al. The mtDNA 15497 G/A polymorphism in cytochrome b in severe obese subjects from Southern Italy. Nutr Metab Cardiovasc Dis. 2006; 16:466–70.
Article73. Okura T, Koda M, Ando F, Niino N, Tanaka M, Shimokata H. Association of the mitochondrial DNA 15497G/A polymorphism with obesity in a middle-aged and elderly Japanese population. Hum Genet. 2003; 113:432–6.
Article74. Chattopadhyay M, Guhathakurta I, Behera P, Ranjan KR, Khanna M, Mukhopadhyay S, et al. Mitochondrial bioenergetics is not impaired in nonobese subjects with type 2 diabetes mellitus. Metabolism. 2011; 60:1702–10.
Article75. Frerman FE, Sabran JL, Taylor JL, Grossberg SE. Leucine catabolism during the differentiation of 3T3-L1 cells. Expression of a mitochondrial enzyme system. J Biol Chem. 1983; 258:7087–93.
Article76. Kitsy A, Carney S, Vivar JC, Knight MS, Pointer MA, Gwathmey JK, et al. Effects of leucine supplementation and serum withdrawal on branched-chain amino acid pathway gene and protein expression in mouse adipocytes. PLoS One. 2014; 9:e102615.
Article77. Kladnicka I, Cedikova M, Kripnerova M, Dvorakova J, Kohoutova M, Tuma Z, et al. Mitochondrial respiration of adipocytes differentiating from human mesenchymal stem cells derived from adipose tissue. Physiol Res. 2019; 68(Suppl 3):S287–96.
Article78. Kusminski CM, Scherer PE. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab. 2012; 23:435–43.
Article79. Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011; 14:537–44.
Article80. Fujiwara M, Tian L, Le PT, DeMambro VE, Becker KA, Rosen CJ, et al. The mitophagy receptor Bcl-2-like protein 13 stimulates adipogenesis by regulating mitochondrial oxidative phosphorylation and apoptosis in mice. J Biol Chem. 2019; 294:12683–94.
Article81. Maniyadath B, Zhang Q, Gupta RK, Mandrup S. Adipose tissue at single-cell resolution. Cell Metab. 2023; 35:386–413.
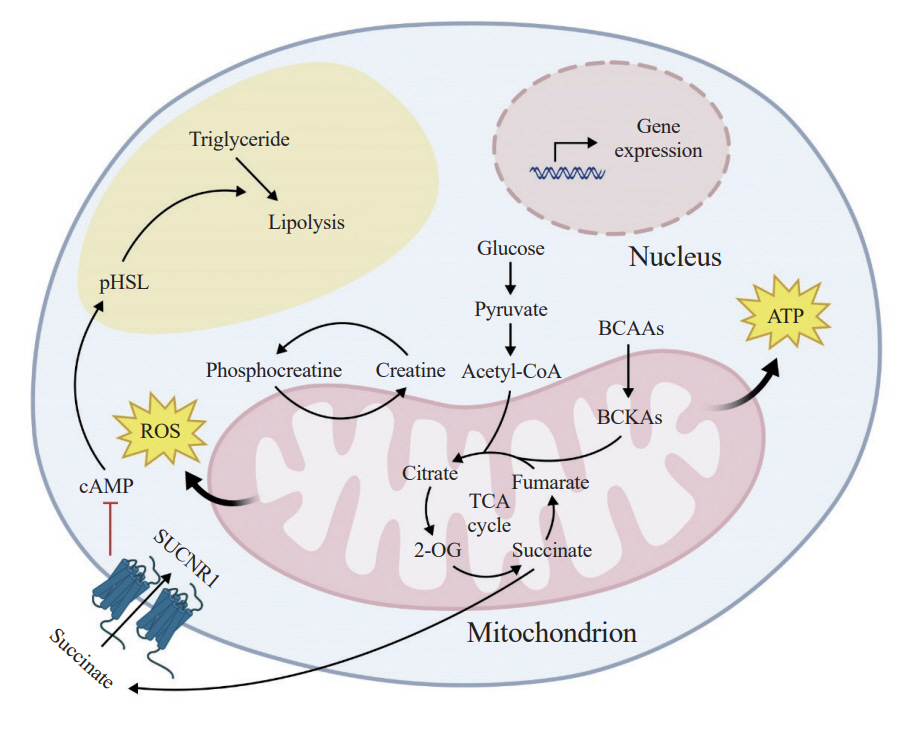
Article82. Shan B, Barker CS, Shao M, Zhang Q, Gupta RK, Wu Y. Multilayered omics reveal sex- and depot-dependent adipose progenitor cell heterogeneity. Cell Metab. 2022; 34:783–99.
Article83. Shao M, Hepler C, Zhang Q, Shan B, Vishvanath L, Henry GH, et al. Pathologic HIF1α signaling drives adipose progenitor dysfunction in obesity. Cell Stem Cell. 2021; 28:685–701.
Article84. Joffin N, Paschoal VA, Gliniak CM, Crewe C, Elnwasany A, Szweda LI, et al. Mitochondrial metabolism is a key regulator of the fibro-inflammatory and adipogenic stromal subpopulations in white adipose tissue. Cell Stem Cell. 2021; 28:702–17.
Article85. Shore AM, Karamitri A, Kemp P, Speakman JR, Graham NS, Lomax MA. Cold-induced changes in gene expression in brown adipose tissue, white adipose tissue and liver. PLoS One. 2013; 8:e68933.
Article86. de Jong JM, Wouters RT, Boulet N, Cannon B, Nedergaard J, Petrovic N. The β3-adrenergic receptor is dispensable for browning of adipose tissues. Am J Physiol Endocrinol Metab. 2017; 312:E508–18.87. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fatlike development of white fat and thermogenesis. Nature. 2012; 481:463–8.
Article88. Scheel AK, Espelage L, Chadt A. Many ways to Rome: exercise, cold exposure and diet: do they all affect BAT activation and WAT browning in the same manner? Int J Mol Sci. 2022; 23:4759.
Article89. Wang F, Xiao F, Du L, Niu Y, Yin H, Zhou Z, et al. Activation of GCN2 in macrophages promotes white adipose tissue browning and lipolysis under leucine deprivation. FASEB J. 2021; 35:e21652.
Article90. Lu X. Maintaining mitochondria in beige adipose tissue. Adipocyte. 2019; 8:77–82.
Article91. Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020; 21:204–24.
Article92. Martinez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020; 11:102.
Article93. Baker SA, Rutter J. Metabolites as signalling molecules. Nat Rev Mol Cell Biol. 2023; 24:355–74.
Article94. Villanueva-Carmona T, Cedo L, Madeira A, Ceperuelo-Mallafre V, Rodriguez-Pena MM, Nunez-Roa C, et al. SUCNR1 signaling in adipocytes controls energy metabolism by modulating circadian clock and leptin expression. Cell Metab. 2023; 35:601–19.
Article95. Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018; 560:102–6.
Article96. Fu T, Sun W, Xue J, Zhou Z, Wang W, Guo Q, et al. Proteolytic rewiring of mitochondria by LONP1 directs cell identity switching of adipocytes. Nat Cell Biol. 2023; 25:848–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- (99m)Tc-MIBI Uptake in a Thyroid Adenoma with Diffuse Adipose Metaplasia
- Mitochondrial Dysfunction in Adipocytes as a Primary Cause of Adipose Tissue Inflammation
- High-fat diet alters the thermogenic gene expression to β-agonists or 18-carbon fatty acids in adipocytes derived from the white and brown adipose tissue of mice
- The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes
- Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders