Anat Cell Biol.
2018 Jun;51(2):119-127. 10.5115/acb.2018.51.2.119.
Ameliorative effects of Moringa on cuprizone-induced memory decline in rat model of multiple sclerosis
- Affiliations
-
- 1Department of Anatomy, Faculty of Basic Medical Sciences, College of Health Sciences, University of Ilorin, Ilorin, Nigeria. gabrielolaiya@yahoo.com
- KMID: 2421176
- DOI: http://doi.org/10.5115/acb.2018.51.2.119
Abstract
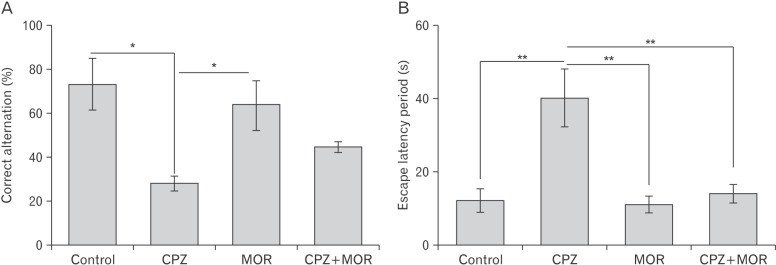
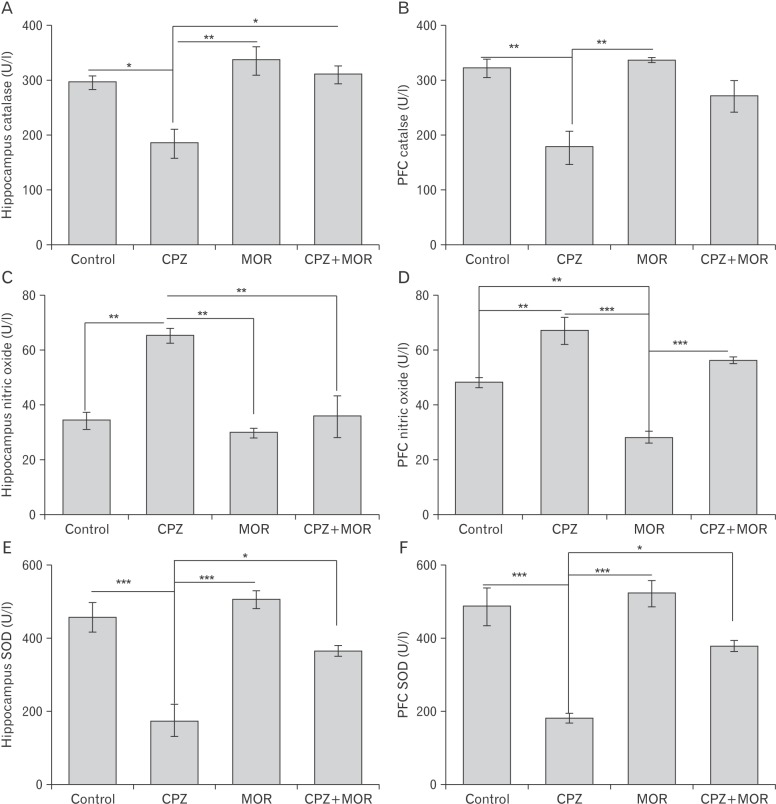
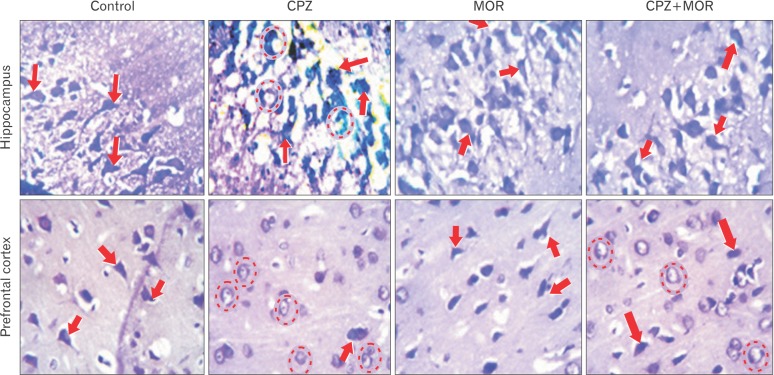
- Cuprizone is a neurotoxin with copper-chelating ability used in animal model of multiple sclerosis in which oxidative stress has been documented as one of the cascade in the pathogenesis. Moringa oleifera is a phytomedicinal plant with antioxidant and neuroprotective properties. This study aimed at evaluating the ameliorative capability of M. oleifera in cuprizone-induced behavioral and histopathological alterations in the prefrontal cortex and hippocampus of Wistar rats. Four groups of rats were treated with normal saline, cuprizone, M. oleifera and a combination of M. oleifera and cuprizone, for five weeks. The rats were subjected to Morris water maze and Y-maze to assess long and short-term memory respectively. The animals were sacrificed, and brain tissues were removed for histochemical and enzyme lysate immunosorbent assay for catalase, superoxide dismutase, and nitric oxide. Cuprizone significantly induced oxidative and nitrosative stress coupled with memory decline and cortico-hippocampal neuronal deficits; however, administration of M. oleifera significantly reversed the neuropathological deficits induced by cuprizone.
Keyword
MeSH Terms
Figure
Reference
-
1. Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000; 47:707–717. PMID: 10852536.2. Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. Lancet Neurol. 2008; 7:841–851. PMID: 18703006.3. Norkute A, Hieble A, Braun A, Johann S, Clarner T, Baumgartner W, Beyer C, Kipp M. Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J Neurosci Res. 2009; 87:1343–1355. PMID: 19021291.4. Praet J, Guglielmetti C, Berneman Z, Van der Linden A, Ponsaerts P. Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev. 2014; 47:485–505. PMID: 25445182.5. Manto M. Abnormal copper homeostasis: mechanisms and roles in neurodegeneration. Toxics. 2014; 2:327–345.6. Skripuletz T, Bussmann JH, Gudi V, Koutsoudaki PN, Pul R, Moharregh-Khiabani D, Lindner M, Stangel M. Cerebellar cortical demyelination in the murine cuprizone model. Brain Pathol. 2010; 20:301–312. PMID: 19371354.7. Skripuletz T, Lindner M, Kotsiari A, Garde N, Fokuhl J, Linsmeier F, Trebst C, Stangel M. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am J Pathol. 2008; 172:1053–1061. PMID: 18349131.8. Draheim T, Liessem A, Scheld M, Wilms F, Weissflog M, Denecke B, Kensler TW, Zendedel A, Beyer C, Kipp M, Wruck CJ, Fragoulis A, Clarner T. Activation of the astrocytic Nrf2/ARE system ameliorates the formation of demyelinating lesions in a multiple sclerosis animal model. Glia. 2016; 64:2219–2230. PMID: 27641725.9. Suneetha A, Raja Rajeswari K. Role of dimethyl fumarate in oxidative stress of multiple sclerosis: a review. J Chromatogr B Analyt Technol Biomed Life Sci. 2016; 1019:15–20.10. Sajjadian M, Kashani IR, Pasbakhsh P, Hassani M, Omidi A, Takzare N, Clarner T, Beyer C, Zendedel A. Protective effects of cannabidiol on cuprizone-induced demyelination in C57BL/6 mice. J Contemp Med Sci. 2017; 3:278–283.11. Carvalho AN, Lim JL, Nijland PG, Witte ME, Van Horssen J. Glutathione in multiple sclerosis: more than just an antioxidant? Mult Scler. 2014; 20:1425–1431. PMID: 24842957.12. World Health Organization. Traditional medicine strategy 2002-2005. Geneva: World Health Organization;2002.13. Eilert U, Wolters B, Nahrstedt A. The antibiotic principle of seeds of Moringa oleifera and Moringa stenopetala. Planta Med. 1981; 42:55–61. PMID: 7255568.14. Ezeamuzie IC, Ambakederemo AW, Shode FO, Ekwebelem SC. Antiinflammatory effects of Moringa oleifera root extract. Int J Pharmacogn. 1996; 34:207–212.15. Gbadamosi IT, Omotoso GO, Olajide OJ, Dada-Habeeb SO, Arogundade TT, Lambe E, Obasi KK. Moringa protects against nicotine-induced morphological and oxidative damage in the frontal cortex of Wistar rats. Anatomy. 2016; 10:170–176.16. Sreelatha S, Jeyachitra A, Padma PR. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol. 2011; 49:1270–1275. PMID: 21385597.17. Ferreira RS, Napoleão TH, Santos AF, Sá RA, Carneiro-da-Cunha MG, Morais MM, Silva-Lucca RA, Oliva ML, Coelho LC, Paiva PM. Coagulant and antibacterial activities of the watersoluble seed lectin from Moringa oleifera. Lett Appl Microbiol. 2011; 53:186–192. PMID: 21605145.18. Libro R, Giacoppo S, Soundara Rajan T, Bramanti P, Mazzon E. Natural phytochemicals in the treatment and prevention of dementia: an overview. Molecules. 2016; 21:518. PMID: 27110749.19. Kasolo JN, Bimenya GS, Ojok L, Ochieng J, Ogwal-okeng JW. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J Med Plants Res. 2010; 4:753–757.20. Pari L, Karamać M, Kosińska A, Rybarczyk A, Amarowicz R. Antioxidant activity of the crude extracts of drumstick tree (Moringa oleifera Lam.) and sweet broomweed (Scoparia dulcis L.) leaves. Pol J Food Nutr Sci. 2007; 57:203–208.21. Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995; 235:207–219. PMID: 7554275.22. Kawada N, Seki S, Inoue M, Kuroki T. Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology. 1998; 27:1265–1274. PMID: 9581680.23. Romanová D, Vachálková A, Cipák L, Ovesná Z, Rauko P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma. 2001; 48:104–107. PMID: 11478688.24. El-Hawary SS, El-Sofany RH, Abdel-Monem AR, Ashour RS, Sleem AA. Polyphenolics content and biological activity of Plectranthus amboinicus (Lour.) spreng growing in Egypt (Lamiaceae). Pharmacogn J. 2012; 2:45–54.25. Ganguly R, Hazra R, Ray K, Guha D. Effect of Moringa oleifera in experimental model of Alzheimer's disease: role of antioxidants. Ann Neurosci. 2005; 12:33–36.26. Lassmann H. What drives disease in multiple sclerosis: inflammation or neurodegeneration? Clin Exp Neuroimmunol. 2010; 1:2–11.27. Lassmann H. Pathology and disease mechanisms in different stages of multiple sclerosis. J Neurol Sci. 2013; 333:1–4. PMID: 23735777.28. Keynes RG, Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Curr Mol Med. 2004; 4:179–191. PMID: 15032712.29. Duda JE, Giasson BI, Chen Q, Gur TL, Hurtig HI, Stern MB, Gollomp SM, Ischiropoulos H, Lee VM, Trojanowski JQ. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am J Pathol. 2000; 157:1439–1445. PMID: 11073803.30. Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, Berente Z, Komoly S, Beyer C. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 2009; 57:807–814. PMID: 19031445.31. Kashani IR, Rajabi Z, Akbari M, Hassanzadeh G, Mohseni A, Eramsadati MK, Rafiee K, Beyer C, Kipp M, Zendedel A. Protective effects of melatonin against mitochondrial injury in a mouse model of multiple sclerosis. Exp Brain Res. 2014; 232:2835–2846. PMID: 24798398.32. Abe H, Saito F, Tanaka T, Mizukami S, Hasegawa-Baba Y, Imatanaka N, Akahori Y, Yoshida T, Shibutani M. Developmental cuprizone exposure impairs oligodendrocyte lineages differentially in cortical and white matter tissues and suppresses glutamatergic neurogenesis signals and synaptic plasticity in the hippocampal dentate gyrus of rats. Toxicol Appl Pharmacol. 2016; 290:10–20. PMID: 26577399.33. Nathoo N, Yong VW, Dunn JF. Understanding disease processes in multiple sclerosis through magnetic resonance imaging studies in animal models. Neuroimage Clin. 2014; 4:743–756. PMID: 24936425.34. Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. Axon physiology. Physiol Rev. 2011; 91:555–602. PMID: 21527732.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synergistic Effect of Coenzyme Q10 and L-Carnitine on Gliosis and Anhedonia, in a Rat Model of Multiple Sclerosis: An Immunohistochemical Study
- Acute Pancreatitis Induced by Moringa Oleifera in a 48 years Old Korean Women: A Case Report
- Immunochemical Study on the Changes of Carbonic anhydrase-II and Iron-binding Proteins in the Demyelinationand and Remyelination model Mouse induced with Cuprizone
- The Decline of Memory Performances of Old Adults and its Correlated Factors
- Effect of Synaptic Loss on Memory in a Neural Network Model