Yonsei Med J.
2017 May;58(3):489-496. 10.3349/ymj.2017.58.3.489.
Development of Inhibitors Targeting Hypoxia-Inducible Factor 1 and 2 for Cancer Therapy
- Affiliations
-
- 1Department of General Surgery, The Hepatosplenic Surgery Center, The First Affiliated Hospital of Harbin Medical University, Harbin, China. k.sun@auckland.ac.nz tangbohmu@163.com
- 2Department of Molecular Medicine & Pathology, Faculty of Medical and Health Sciences, The University of Auckland, Auckland, New Zealand.
- KMID: 2419104
- DOI: http://doi.org/10.3349/ymj.2017.58.3.489
Abstract
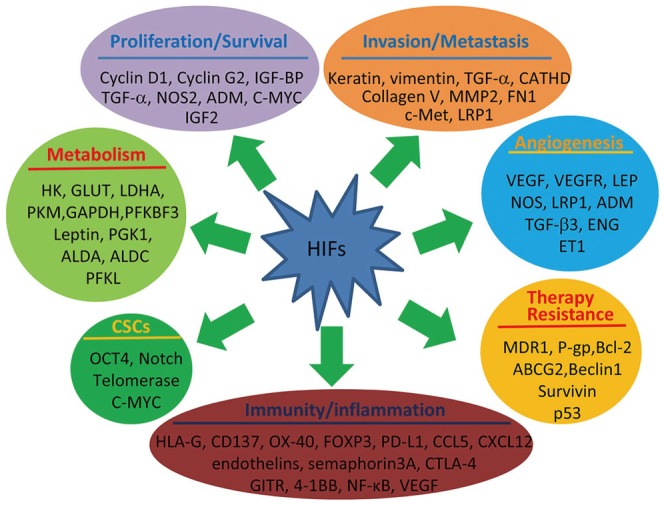
- Hypoxia is frequently observed in solid tumors and also one of the major obstacles for effective cancer therapies. Cancer cells take advantage of their ability to adapt hypoxia to initiate a special transcriptional program that renders them more aggressive biological behaviors. Hypoxia-inducible factors (HIFs) are the key factors that control hypoxia-inducible pathways by regulating the expression of a vast array of genes involved in cancer progression and treatment resistance. HIFs, mainly HIF-1 and -2, have become potential targets for developing novel cancer therapeutics. This article reviews the updated information in tumor HIF pathways, particularly recent advances in the development of HIF inhibitors. These inhibitors interfere with mRNA expression, protein synthesis, protein degradation and dimerization, DNA binding and transcriptional activity of HIF-1 and -2, or both. Despite efforts in the past two decades, no agents directly inhibiting HIFs have been approved for treating cancer patients. By analyzing results of the published reports, we put the perspectives at the end of the article. The therapeutic efficacy of HIF inhibitors may be improved if more efforts are devoted on developing agents that are able to simultaneously target HIF-1 and -2, increasing the penetrating capacity of HIF inhibitors, and selecting suitable patient subpopulations for clinical trials.
MeSH Terms
-
Antineoplastic Agents/*pharmacology
Basic Helix-Loop-Helix Transcription Factors/genetics/*physiology
Genes, Tumor Suppressor/physiology
Humans
Hypoxia-Inducible Factor 1/genetics/*physiology
Hypoxia-Inducible Factor 1, alpha Subunit/*antagonists & inhibitors/metabolism
Neoplasms/*drug therapy/genetics/pathology/*therapy
RNA, Messenger/genetics
Transcriptional Activation
Antineoplastic Agents
Basic Helix-Loop-Helix Transcription Factors
Hypoxia-Inducible Factor 1
Hypoxia-Inducible Factor 1, alpha Subunit
RNA, Messenger
Figure
Reference
-
1. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002; 2:38–47. PMID: 11902584.2. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011; 11:393–410. PMID: 21606941.
Article3. Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008; 15:678–685. PMID: 18259193.
Article4. Triner D, Shah YM. Hypoxia-inducible factors: a central link between inflammation and cancer. J Clin Invest. 2016; 126:3689–3698. PMID: 27525434.
Article5. Onnis B, Rapisarda A, Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med. 2009; 13:2780–2786. PMID: 19674190.
Article6. Koh MY, Powis G. Passing the baton: the HIF switch. Trends Biochem Sci. 2012; 37:364–372. PMID: 22818162.
Article7. Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001; 93:266–276. PMID: 11181773.8. Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007; 26:225–239. PMID: 17440684.
Article9. Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008; 8:967–975. PMID: 18987634.
Article10. Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998; 7:205–213. PMID: 9840812.11. Semenza GL. The hypoxic tumor microenvironment: a driving force for breast cancer progression. Biochim Biophys Acta. 2016; 1863:382–391. PMID: 26079100.
Article12. Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011; 12:9–22. PMID: 22169972.
Article13. Duan C. Hypoxia-inducible factor 3 biology: complexities and emerging themes. Am J Physiol Cell Physiol. 2016; 310:C260–C269. PMID: 26561641.
Article14. Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J Biol Chem. 2001; 276:12645–12653. PMID: 11278891.15. Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001; 294:1337–1340. PMID: 11598268.
Article16. Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors--similar but not identical. Mol Cells. 2010; 29:435–442. PMID: 20396958.17. Zhao D, Zhai B, He C, Tan G, Jiang X, Pan S, et al. Upregulation of HIF-2α induced by sorafenib contributes to the resistance by activating the TGF-α/EGFR pathway in hepatocellular carcinoma cells. Cell Signal. 2014; 26:1030–1039. PMID: 24486412.
Article18. Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006; 10:413–423. PMID: 17097563.19. Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015; 5:378–389. PMID: 26579469.
Article20. Unwith S, Zhao H, Hennah L, Ma D. The potential role of HIF on tumour progression and dissemination. Int J Cancer. 2015; 136:2491–2503. PMID: 24729302.
Article21. Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem. 2012; 49:24–40. PMID: 22305612.
Article22. Yang YJ, Na HJ, Suh MJ, Ban MJ, Byeon HK, Kim WS, et al. Hypoxia induces epithelial-mesenchymal transition in follicular thyroid cancer: involvement of regulation of twist by hypoxia inducible factor-1α. Yonsei Med J. 2015; 56:1503–1514. PMID: 26446630.
Article23. Peng G, Liu Y. Hypoxia-inducible factors in cancer stem cells and inflammation. Trends Pharmacol Sci. 2015; 36:374–383. PMID: 25857287.
Article24. Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A. 2014; 111:E5429–E5438. PMID: 25453096.
Article25. Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat. 2011; 14:191–201. PMID: 21466972.
Article26. Wigerup C, Påhlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016; 164:152–169. PMID: 27139518.
Article27. Warfel NA, El-Deiry WS. HIF-1 signaling in drug resistance to chemotherapy. Curr Med Chem. 2014; 21:3021–3028. PMID: 24735366.
Article28. Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. 2016; 138:1058–1066. PMID: 25784597.
Article29. Kim JW, Gao P, Dang CV. Effects of hypoxia on tumor metabolism. Cancer Metastasis Rev. 2007; 26:291–298. PMID: 17415528.
Article30. Jeong W, Rapisarda A, Park SR, Kinders RJ, Chen A, Melillo G, et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1α), in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2014; 73:343–348. PMID: 24292632.
Article31. Greenberger LM, Horak ID, Filpula D, Sapra P, Westergaard M, Frydenlund HF, et al. A RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968, inhibits tumor cell growth. Mol Cancer Ther. 2008; 7:3598–3608. PMID: 18974394.32. Coltella N, Valsecchi R, Ponente M, Ponzoni M, Bernardi R. Synergistic leukemia eradication by combined treatment with retinoic acid and HIF inhibition by EZN-2208 (PEG-SN38) in preclinical models of PML-RARα and PLZF-RARα-driven leukemia. Clin Cancer Res. 2015; 21:3685–3694. PMID: 25931453.
Article33. Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004; 64:1475–1482. PMID: 14983893.34. Lee K, Kim HM. A novel approach to cancer therapy using PX-478 as a HIF-1α inhibitor. Arch Pharm Res. 2011; 34:1583–1585. PMID: 22076756.
Article35. Liu X, Chen Z, Xu C, Leng X, Cao H, Ouyang G, et al. Repression of hypoxia-inducible factor α signaling by Set7-mediated methylation. Nucleic Acids Res. 2015; 43:5081–5098. PMID: 25897119.
Article36. Ma L, Li G, Zhu H, Dong X, Zhao D, Jiang X, et al. 2-methoxyestradiol synergizes with sorafenib to suppress hepatocellular carcinoma by simultaneously dysregulating hypoxia-inducible factor-1 and -2. Cancer Lett. 2014; 355:96–105. PMID: 25218350.
Article37. Narita T, Yin S, Gelin CF, Moreno CS, Yepes M, Nicolaou KC, et al. Identification of a novel small molecule HIF-1alpha translation inhibitor. Clin Cancer Res. 2009; 15:6128–6136. PMID: 19789328.38. Lee SH, Jee JG, Bae JS, Liu KH, Lee YM. A group of novel HIF-1α inhibitors, glyceollins, blocks HIF-1α synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 binding. J Cell Physiol. 2015; 230:853–862. PMID: 25204544.
Article39. Hu N, Jiang D, Huang E, Liu X, Li R, Liang X, et al. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J Cell Sci. 2013; 126(Pt 2):532–541. PMID: 23203800.
Article40. Newman B, Liu Y, Lee HF, Sun D, Wang Y. HSP90 inhibitor 17-AAG selectively eradicates lymphoma stem cells. Cancer Res. 2012; 72:4551–4561. PMID: 22751135.
Article41. Ibrahim NO, Hahn T, Franke C, Stiehl DP, Wirthner R, Wenger RH, et al. Induction of the hypoxia-inducible factor system by low levels of heat shock protein 90 inhibitors. Cancer Res. 2005; 65:11094–11100. PMID: 16322259.
Article42. Kubo T, Maezawa N, Osada M, Katsumura S, Funae Y, Imaoka S. Bisphenol A, an environmental endocrine-disrupting chemical, inhibits hypoxic response via degradation of hypoxia-inducible factor 1alpha (HIF-1alpha): structural requirement of bisphenol A for degradation of HIF-1alpha. Biochem Biophys Res Commun. 2004; 318:1006–1011. PMID: 15147973.43. Ellinghaus P, Heisler I, Unterschemmann K, Haerter M, Beck H, Greschat S, et al. BAY 87-2243, a highly potent and selective inhibitor of hypoxia-induced gene activation has antitumor activities by inhibition of mitochondrial complex I. Cancer Med. 2013; 2:611–624. PMID: 24403227.44. Dat NT, Jin X, Lee JH, Lee D, Hong YS, Lee K, et al. Abietane diterpenes from Salvia miltiorrhiza inhibit the activation of hypoxiainducible factor-1. J Nat Prod. 2007; 70:1093–1097. PMID: 17583950.
Article45. Nakazawa MS, Eisinger-Mathason TS, Sadri N, Ochocki JD, Gade TP, Amin RK, et al. Epigenetic re-expression of HIF-2α suppresses soft tissue sarcoma growth. Nat Commun. 2016; 7:10539. PMID: 26837714.
Article46. Lee K, Kang JE, Park SK, Jin Y, Chung KS, Kim HM, et al. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem Pharmacol. 2010; 80:982–989. PMID: 20599784.47. Cui XY, Tinholt M, Stavik B, Dahm AE, Kanse S, Jin Y, et al. Effect of hypoxia on tissue factor pathway inhibitor expression in breast cancer. J Thromb Haemost. 2016; 14:387–396. PMID: 26598923.
Article48. Raninga PV, Di Trapani G, Vuckovic S, Bhatia M, Tonissen KF. Inhibition of thioredoxin 1 leads to apoptosis in drug-resistant multiple myeloma. Oncotarget. 2015; 6:15410–15424. PMID: 25945832.
Article49. Jordan BF, Runquist M, Raghunand N, Gillies RJ, Tate WR, Powis G, et al. The thioredoxin-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) decreases vascular permeability in tumor xenografts monitored by dynamic contrast enhanced magnetic resonance imaging. Clin Cancer Res. 2005; 11(2 Pt 1):529–536. PMID: 15701837.50. Miranda E, Nordgren IK, Male AL, Lawrence CE, Hoakwie F, Cuda F, et al. A cyclic peptide inhibitor of HIF-1 heterodimerization that inhibits hypoxia signaling in cancer cells. J Am Chem Soc. 2013; 135:10418–10425. PMID: 23796364.
Article51. Scheuermann TH, Li Q, Ma HW, Key J, Zhang L, Chen R, et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat Chem Biol. 2013; 9:271–276. PMID: 23434853.
Article52. Scheuermann TH, Tomchick DR, Machius M, Guo Y, Bruick RK, Gardner KH. Artificial ligand binding within the HIF2alpha PAS-B domain of the HIF2 transcription factor. Proc Natl Acad Sci U S A. 2009; 106:450–455. PMID: 19129502.53. Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci U S A. 2009; 106:17910–17915. PMID: 19805192.
Article54. Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005; 65:9047–9055. PMID: 16204079.
Article55. Yu W, Denu RA, Krautkramer KA, Grindle KM, Yang DT, Asimakopoulos F, et al. Loss of SIRT3 provides growth advantage for B cell malignancies. J Biol Chem. 2016; 291:3268–3279. PMID: 26631723.
Article56. Young RM, Wang SJ, Gordan JD, Ji X, Liebhaber SA, Simon MC. Hypoxia-mediated selective mRNA translation by an internal ribosome entry site-independent mechanism. J Biol Chem. 2008; 283:16309–16319. PMID: 18430730.
Article57. Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW. Gene transfer of antisense hypoxia inducible factor-1 alpha enhances the therapeutic efficacy of cancer immunotherapy. Gene Ther. 2001; 8:638–645. PMID: 11320410.58. Bertozzi D, Marinello J, Manzo SG, Fornari F, Gramantieri L, Capranico G. The natural inhibitor of DNA topoisomerase I, camptothecin, modulates HIF-1α activity by changing miR expression patterns in human cancer cells. Mol Cancer Ther. 2014; 13:239–248. PMID: 24252850.
Article59. Fu B, Xue J, Li Z, Shi X, Jiang BH, Fang J. Chrysin inhibits expression of hypoxia-inducible factor-1alpha through reducing hypoxia-inducible factor-1alpha stability and inhibiting its protein synthesis. Mol Cancer Ther. 2007; 6:220–226. PMID: 17237281.60. Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxiainducible factor-1 alpha-degradative pathway. J Biol Chem. 2002; 277:29936–29944. PMID: 12052835.61. Bohonowych JE, Peng S, Gopal U, Hance MW, Wing SB, Argraves KM, et al. Comparative analysis of novel and conventional Hsp90 inhibitors on HIF activity and angiogenic potential in clear cell renal cell carcinoma: implications for clinical evaluation. BMC Cancer. 2011; 11:520. PMID: 22172030.
Article62. Hutt DM, Roth DM, Vignaud H, Cullin C, Bouchecareilh M. The histone deacetylase inhibitor, Vorinostat, represses hypoxia inducible factor 1 alpha expression through translational inhibition. PLoS One. 2014; 9:e106224. PMID: 25166596.
Article63. Lee K, Qian DZ, Rey S, Wei H, Liu JO, Semenza GL. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci U S A. 2009; 106:2353–2358. PMID: 19168635.
Article64. Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004; 6:33–43. PMID: 15261140.
Article65. Minegishi H, Fukashiro S, Ban HS, Nakamura H. Discovery of indenopyrazoles as a new class of hypoxia inducible factor (HIF)-1 inhibitors. ACS Med Chem Lett. 2013; 4:297–301. PMID: 24900662.
Article66. Santos AI, Carreira BP, Nobre RJ, Carvalho CM, Araújo IM. Stimulation of neural stem cell proliferation by inhibition of phosphodiesterase 5. Stem Cells Int. 2014; 2014:878397. PMID: 24550991.
Article67. Moreno-Manzano V, Rodríguez-Jiménez FJ, Aceña-Bonilla JL, Fustero-Lardíes S, Erceg S, Dopazo J, et al. FM19G11, a new hypoxia-inducible factor (HIF) modulator, affects stem cell differentiation status. J Biol Chem. 2010; 285:1333–1342. PMID: 19897487.
Article68. Koh MY, Lemos R Jr, Liu X, Powis G. The hypoxia-associated factor switches cells from HIF-1α- to HIF-2α-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011; 71:4015–4027. PMID: 21512133.
Article69. Seton-Rogers S. Hypoxia: HIF switch. Nat Rev Cancer. 2011; 11:391. PMID: 21606940.70. Chau NM, Rogers P, Aherne W, Carroll V, Collins I, McDonald E, et al. Identification of novel small molecule inhibitors of hypoxiainducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1alpha induction in response to hypoxic stress and growth factors. Cancer Res. 2005; 65:4918–4928. PMID: 15930314.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxia-inducible factor 1, hepatocellular carcinoma and angiogenesis
- From tumor hypoxia to cancer progression: the implications of hypoxia-inducible factor-1 expression in cancers
- HIF-1alpha Upregulation due to Depletion of the Free Ubiquitin Pool
- Recent Progress in Synthesis of99m Tc‑labeled Complexes with Nitroimidazoles as SPECT Probes for Targeting Tumor Hypoxia
- CaMKII Inhibitor KN-62 Blunts Tumor Response to Hypoxia by Inhibiting HIF-1alpha in Hepatoma Cells