J Korean Med Sci.
2015 Oct;30(10):1388-1395. 10.3346/jkms.2015.30.10.1388.
HIF-1alpha Upregulation due to Depletion of the Free Ubiquitin Pool
- Affiliations
-
- 1Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea. parkjw@snu.ac.kr
- 2Department of Pharmacology, Seoul National University College of Medicine, Seoul, Korea.
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2344170
- DOI: http://doi.org/10.3346/jkms.2015.30.10.1388
Abstract
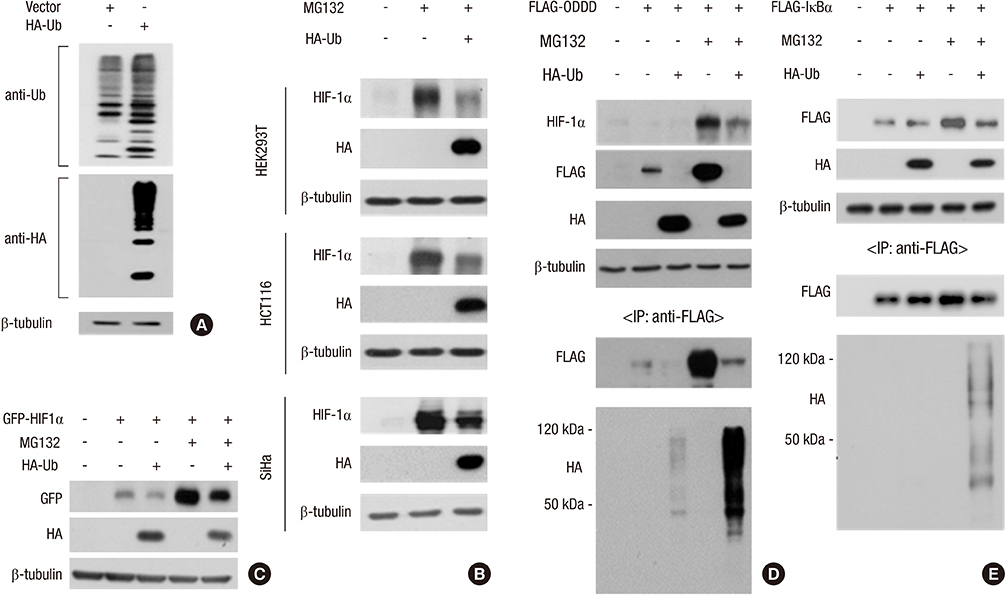
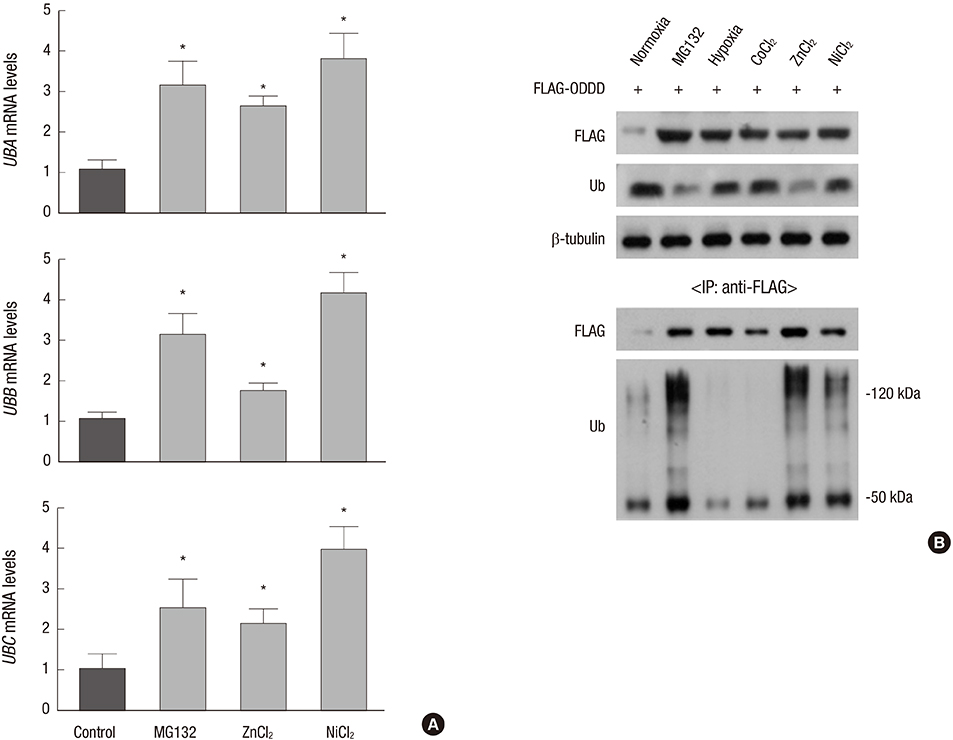
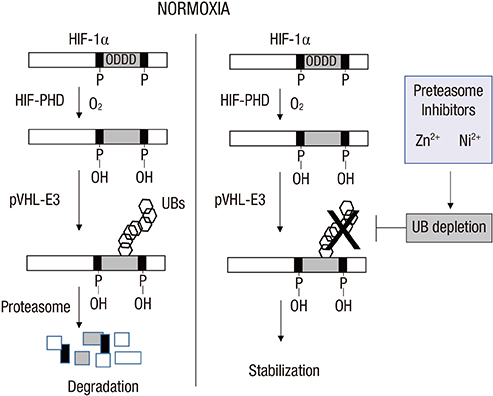
- Hypoxia-inducible factor 1alpha (HIF-1alpha), which transactivates a variety of hypoxia-induced genes, is rapidly degraded under nomoxia through the hydroxylation-ubiquitination-proteasome pathway. In this study, we addressed how HIF-1alpha is stabilized by proteasome inhibitors. The ubiquitin pool was rapidly reduced after proteasome inhibition, followed by the accumulation of non-ubiquitinated HIF-1alpha. The poly-ubiquitination of HIF-1alpha was resumed by restoration of free ubiquitin, which suggests that the HIF-1alpha stabilization under proteasome inhibition is attributed to depletion of the free ubiquitin pool. Ni2+ and Zn2+ also stabilized HIF-1alpha with depletion of the free ubiquitin pool and these effects of metal ions were attenuated by restoration of free ubiquitin. Ni2+ and Zn2+ may disturb the recycling of free ubiquitin, as MG132 does. Based on these results, the state of the ubiquitin pool seems to be another critical factor determining the cellular level of HIF-1alpha.
Keyword
MeSH Terms
-
Cell Hypoxia/physiology
Cell Line, Tumor
HCT116 Cells
HEK293 Cells
Humans
Hypoxia-Inducible Factor 1, alpha Subunit/biosynthesis/*metabolism
Leupeptins/pharmacology
Nickel/chemistry
Proteasome Endopeptidase Complex/*metabolism
Proteasome Inhibitors/*pharmacology
Ubiquitin/*metabolism
Ubiquitination/*physiology
Up-Regulation
Zinc/chemistry
Hypoxia-Inducible Factor 1, alpha Subunit
Leupeptins
Nickel
Proteasome Endopeptidase Complex
Proteasome Inhibitors
Ubiquitin
Zinc
Figure
Reference
-
1. Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001; 2:169–178.2. Groothuis TA, Dantuma NP, Neefjes J, Salomons FA. Ubiquitin crosstalk connecting cellular processes. Cell Div. 2006; 1:21.3. Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000; 19:94–102.4. Park CW, Ryu KY. Cellular ubiquitin pool dynamics and homeostasis. BMB Rep. 2014; 47:475–482.5. Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009; 10:550–563.6. Brahimi-Horn C, Pouyssegur J. When hypoxia signalling meets the ubiquitin-proteasomal pathway, new targets for cancer therapy. Crit Rev Oncol Hematol. 2005; 53:115–123.7. Chun YS, Kim MS, Park JW. Oxygen-dependent and -independent regulation of HIF-1alpha. J Korean Med Sci. 2002; 17:581–588.8. Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004; 5:343–354.9. Kallio PJ, Wilson WJ, O'Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem. 1999; 274:6519–6525.10. Shin DH, Li SH, Chun YS, Huang LE, Kim MS, Park JW. CITED2 mediates the paradoxical responses of HIF-1alpha to proteasome inhibition. Oncogene. 2008; 27:1939–1944.11. Chun YS, Choi E, Kim GT, Lee MJ, Lee MJ, Lee SE, Kim MS, Park JW. Zinc induces the accumulation of hypoxia-inducible factor (HIF)-1alpha, but inhibits the nuclear translocation of HIF-1beta, causing HIF-1 inactivation. Biochem Biophys Res Commun. 2000; 268:652–656.12. Wang GC, Hazra TK, Mitra S, Lee HM, Englander EW. Mitochondrial DNA damage and a hypoxic response are induced by CoCI2 in rat neuronal PC12 cells. Nucleic Acids Res. 2000; 28:2135–2140.13. Salnikow K, An WG, Melillo G, Blagosklonny MV, Costa M. Nickel-induced transformation shifts the balance between HIF-1 and p53 transcription factors. Carcinogenesis. 1999; 20:1819–1823.14. Xu Q, Farah M, Webster JM, Wojcikiewicz RJ. Bortezomib rapidly suppresses ubiquitin thiolesterification to ubiquitin-conjugating enzymes and inhibits ubiquitination of histones and type I inositol 1,4,5-trisphosphate receptor. Mol Cancer Ther. 2004; 3:1263–1269.15. Melikova MS, Kondratov KA, Kornilova ES. Two different stages of epidermal growth factor (EGF) receptor endocytosis are sensitive to free ubiquitin depletion produced by proteasome inhibitor MG132. Cell Biol Int. 2006; 30:31–43.16. Kim I, Kim CH, Kim JH, Lee J, Choi JJ, Chen ZA, Lee MG, Chung KC, Hsu CY, Ahn YS. Pyrrolidine dithiocarbamate and zinc inhibit proteasome-dependent proteolysis. Exp Cell Res. 2004; 298:229–238.17. Qiu M, Chen Y, Chu Y, Song S, Yang N, Gao J, Wu Z. Zinc ionophores pyrithione inhibits herpes simplex virus replication through interfering with proteasome function and NF-kappaB activation. Antiviral Res. 2013; 100:44–53.18. Cvek B, Milacic V, Taraba J, Dou QP. Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J Med Chem. 2008; 51:6256–6258.19. Frezza M, Hindo SS, Tomco D, Allard MM, Cui QC, Heeg MJ, Chen D, Dou QP, Verani CN. Comparative activities of nickel(II) and zinc(II) complexes of asymmetric [NN'O] ligands as 26S proteasome inhibitors. Inorg Chem. 2009; 48:5928–5937.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phospholipase D2 promotes degradation of hypoxia-inducible factor-1alpha independent of lipase activity
- STAT3 inhibits the degradation of HIF-1alpha by pVHL-mediated ubiquitination
- HIF-1alpha: a Valid Therapeutic Target for Tumor Therapy
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Transitional Bladder Cancer
- Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions