Allergy Asthma Immunol Res.
2018 Sep;10(5):533-542. 10.4168/aair.2018.10.5.533.
Impact of the Endothelial Tight Junction Protein Claudin-5 on Clinical Profiles of Patients With COPD
- Affiliations
-
- 1Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea. jas877@schmc.ac.kr
- KMID: 2418050
- DOI: http://doi.org/10.4168/aair.2018.10.5.533
Abstract
- PURPOSE
The tight junction protein claudin-5 (CLDN5) is critical to the control of endothelial cellular polarity and pericellular permeability. The role of CLDN5 in chronic obstructive pulmonary disease (COPD) remains unclear. The aim of this study was to investigate the association between CLDN5 levels and clinical variables in patients with COPD.
METHODS
In total, 30 patients with COPD and 30 healthy controls were enrolled in the study. The plasma CLDN5 level was checked in patients with stable or exacerbated COPD and in healthy controls.
RESULTS
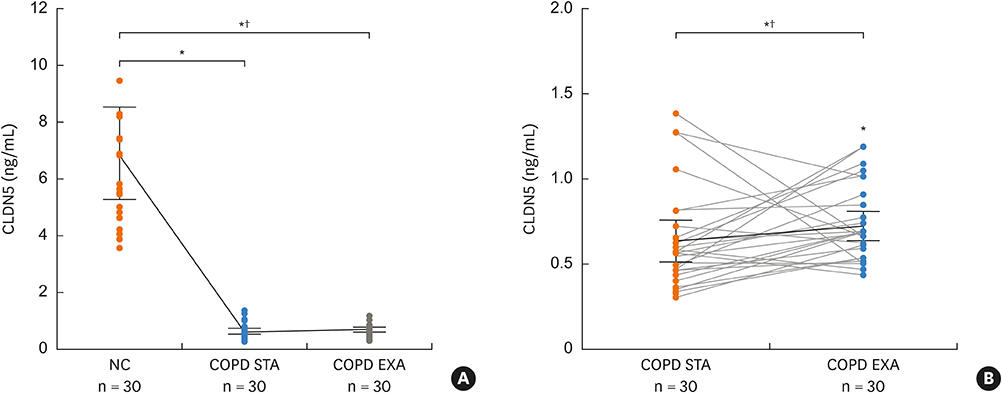
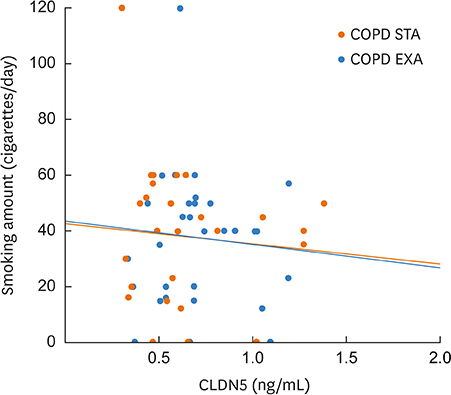
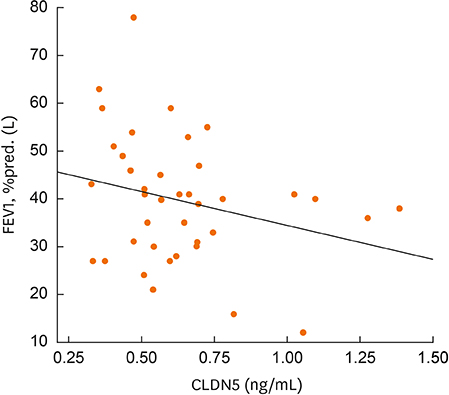
The mean plasma CLDN5 level of patients with COPD was 0.63 ± 0.05 ng/mL and that of healthy controls was 6.9 ± 0.78 ng/mL (P = 0.001). The mean plasma CLDN5 level was 0.71 ± 0.05 ng/mL in exacerbated COPD patients and 0.63 ± 0.04 ng/mL in patients with stable COPD (P < 0.05). The plasma CLDN5 level among COPD subjects was correlated with the smoking amount (r = −0.530, P = 0.001). The plasma CLDN5 level in stable COPD patients was correlated with forced expiratory volume in one second (FEV1, %pred.) (r = −0.481, P = 0.037).
CONCLUSIONS
The plasma CLDN5 level was not correlated with age. CLDN5 may be involved in the pathogenesis of COPD. Further studies having a larger sample size will be needed to clarify CLDN5 in COPD.
Keyword
MeSH Terms
Figure
Reference
-
1. Schlingmann B, Molina SA, Koval M. Claudins: gatekeepers of lung epithelial function. Semin Cell Dev Biol. 2015; 42:47–57.
Article2. Soini Y. Claudins in lung diseases. Respir Res. 2011; 12:70.
Article3. Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003; 285:L1166–L1178.
Article4. Kaarteenaho-Wiik R, Soini Y. Claudin-1, -2, -3, -4, -5, and -7 in usual interstitial pneumonia and sarcoidosis. J Histochem Cytochem. 2009; 57:187–195.
Article5. Lappi-Blanco E, Lehtonen ST, Sormunen R, Merikallio HM, Soini Y, Kaarteenaho RL. Divergence of tight and adherens junction factors in alveolar epithelium in pulmonary fibrosis. Hum Pathol. 2013; 44:895–907.
Article6. Jang AS, Concel VJ, Bein K, Brant KA, Liu S, Pope-Varsalona H, et al. Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am J Respir Cell Mol Biol. 2011; 44:483–490.
Article7. Li H, Singh S, Potula R, Persidsky Y, Kanmogne GD. Dysregulation of claudin-5 in HIV-induced interstitial pneumonitis and lung vascular injury. Protective role of peroxisome proliferator-activated receptor-γ. Am J Respir Crit Care Med. 2014; 190:85–97.
Article8. Moon KY, Lee PH, Kim BG, Park CS, Leikauf GD, Jang AS. Claudin 5 in a murine model of allergic asthma: Its implication and response to steroid treatment. J Allergy Clin Immunol. 2015; 136:1694–1696.e5.
Article9. Jin HJ, Park HS. Claudin may be a potential biomarker for epithelial barrier dysfunction in asthma. Allergy Asthma Immunol Res. 2018; 10:4–5.
Article10. Lee PH, Kim BG, Lee SH, Lee JH, Park SW, Kim DJ, et al. Alteration in claudin-4 contributes to airway inflammation and responsiveness in asthma. Allergy Asthma Immunol Res. 2018; 10:25–33.
Article11. Donaldson GC, Seemungal TA, Patel IS, Bhowmik A, Wilkinson TM, Hurst JR, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005; 128:1995–2004.
Article12. Yoo KH, Ahn HR, Park JK, Kim JW, Nam GH, Hong SK, et al. Burden of respiratory disease in Korea: an observational study on allergic rhinitis, asthma, COPD, and rhinosinusitis. Allergy Asthma Immunol Res. 2016; 8:527–534.
Article13. Perera PN, Armstrong EP, Sherrill DL, Skrepnek GH. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD. 2012; 9:131–141.
Article14. Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009; 179:369–374.
Article15. Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011; 365:1184–1192.
Article16. Global Initiative for Chronic Obstructive Lung Disease (GOLD) [Internet]. place unknown: Global Initiative for Chronic Obstructive Lung Disease;2015. cited 2015 Dec 15. Available from: http://goldcopd.org/.17. Moon KY, Lee PH, Park SW, Park CS, Jang AS. Serum angiopoietin is associated with lung function in patients with asthma: a retrospective cohort study. BMC Pulm Med. 2014; 14:143.
Article18. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187:347–365.19. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.20. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Eur Respir J Suppl. 1993; 16:Suppl 16. 5–40.21. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006; 355:763–778.
Article22. McGee DL. Diverse Populations Collaboration. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005; 15:87–97.
Article23. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002; 288:1723–1727.
Article24. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001; 2:285–293.
Article25. Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004; 5:261–270.
Article26. Chen X, Gumbiner BM. Crosstalk between different adhesion molecules. Curr Opin Cell Biol. 2006; 18:572–578.
Article27. Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003; 161:653–660.
Article28. Ohta H, Chiba S, Ebina M, Furuse M, Nukiwa T. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L193–L205.
Article29. Daugherty BL, Mateescu M, Patel AS, Wade K, Kimura S, Gonzales LW, et al. Developmental regulation of claudin localization by fetal alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L1266–L1273.
Article30. World Health Organization (WHO). World health statistics 2008 [Internet]. Geneva, Switzerland: World Health Organization;2008. cited 2012 Sep 11. Available from: http://www.who.int/whosis/whostat/EN_WHS08_Full.pdf.31. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD [Internet]. place unknown: Global Initiative for Chronic Obstructive Lung Disease;2010. cited 2012 Sep 12. Available from: http://www.goldcopd.org/uploads/users/files/GOLDReport_April112011.pdf.32. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005; 60:925–931.
Article33. Mallia P, Footitt J, Sotero R, Jepson A, Contoli M, Trujillo-Torralbo MB, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186:1117–1124.
Article34. Suzuki R, Nakamura Y, Chiba S, Mizuno T, Abe K, Horii Y, et al. Mitigation of tight junction protein dysfunction in lung microvascular endothelial cells with pitavastatin. Pulm Pharmacol Ther. 2016; 38:27–35.
Article35. Hill AT, Bayley D, Stockley RA. The interrelationship of sputum inflammatory markers in patients with chronic bronchitis. Am J Respir Crit Care Med. 1999; 160:893–898.
Article36. Chen W, Sharma R, Rizzo AN, Siegler JH, Garcia JG, Jacobson JR. Role of claudin-5 in the attenuation of murine acute lung injury by simvastatin. Am J Respir Cell Mol Biol. 2014; 50:328–336.
Article37. Baum SL, Andreson IG, Baker RR, Rowlands CC. Electron spin resonance and spin trap investigation of free radicals in cigarette smoke: development of a quantification procedure. Anal Chim Acta. 2003; 481:1–13.
Article38. Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur Respir J. 2012; 39:419–428.
Article39. Olivera DS, Boggs SE, Beenhouwer C, Aden J, Knall C. Cellular mechanisms of mainstream cigarette smoke-induced lung epithelial tight junction permeability changes in vitro . Inhal Toxicol. 2007; 19:13–22.40. Veljkovic E, Jiricny J, Menigatti M, Rehrauer H, Han W. Chronic exposure to cigarette smoke condensate in vitro induces epithelial to mesenchymal transition-like changes in human bronchial epithelial cells, BEAS-2B. Toxicol In Vitro. 2011; 25:446–453.41. Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004; 59:574–580.
Article42. Clark CJ, Cochrane LM, Mackay E, Paton B. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur Respir J. 2000; 15:92–97.
Article43. Park HJ, Byun MK, Kim HJ, Ahn CM, Lee JH, Shin KC, et al. Asthma-COPD overlap shows favorable clinical outcomes compared to pure COPD in a Korean COPD cohort. Allergy Asthma Immunol Res. 2017; 9:431–437.
Article44. Kim YS, Choi JP, Kim MH, Park HK, Yang S, Kim YS, et al. IgG sensitization to extracellular vesicles in indoor dust is closely associated with the prevalence of non-eosinophilic asthma, COPD, and lung cancer. Allergy Asthma Immunol Res. 2016; 8:198–205.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of ozone on circulating tight junction protein claudin 4 and claudin 5 in patients with asthma
- Significance of the Decreased Expressions of Claudin-1 and ZO-1 Protein as Prognostic Factors in Breast Cancer
- Indol-3-Carbinol Regulated Tight Junction Permeability and Associated-Protein Level and Suppressed Cell Invasion in Human Colon Cancer Cell Line, HT-29
- Chlorogenic Acid Decreases Retinal Vascular Hyperpermeability in Diabetic Rat Model
- Effects of High Glucose and Dexamethasone on the Permeability in Trabecular Meshwork Cells