Ewha Med J.
2018 Jul;41(3):53-62. 10.12771/emj.2018.41.3.53.
Microarray Analysis in Pulmonary Hypertensive Rat Heart after Simvastatin Treatment
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University College of Medicine, Seoul, Korea. ymhong@ewha.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Ewha Womans University College of Medicine, Seoul, Korea.
- KMID: 2417942
- DOI: http://doi.org/10.12771/emj.2018.41.3.53
Abstract
OBJECTIVES
Simvastatin has been reported to attenuate the development of pulmonary hypertension through increased apoptosis as well as reduced proliferation of smooth muscle cells in obstructive vascular lesions. Microarray experiment can accomplish many genetic tests in parallel. The purpose of this study is to evaluate altered expressions of gene in rat hearts with monocrotaline (MCT)-induced pulmonary arterial hypertension after simvastatin treatment.
METHODS
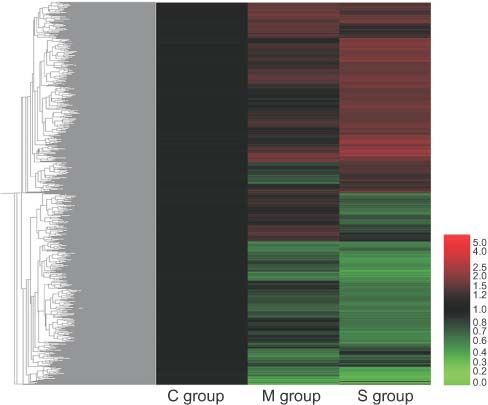
Six-week-old male rats were grouped as follows: control group (C group, saline injection), M group (MCT 60 mg/kg), and S group (MCT 60 mg/kg plus 10 mg/kg/day simvastatin by gavage during 28 days). Body weight, right ventricular pressure and right ventricular/left ventricle+septum ratio in each group were measured. The rats were sacrificed after 28 days. Total RNA was extracted from the rat heart tissue and microarray analysis was performed.
RESULTS
Administration of simvastatin significantly inhibited the progression of right ventricular hypertrophy at day 28 in the S group than in the M group. Compared with the C group, MCT was associated with a significant difference in expression of genes related to biosynthesis and with the regulation of heart contraction rate. Simvastatin treatment resulted in a significantly changed expression of genes about the regulation of progression through cell cycle and system development compared to the M group. The expressions of nitric oxide synthase and brain natriuretic peptide were significantly decreased after simvastatin treatment.
CONCLUSION
Administration of simvastatin exerted inhibitory effects on right ventricular hypertrophy during the development of MCT-induced pulmonary arterial hypertension in rats. Simvastatin changes the expression of genes associated with various functions.
Keyword
MeSH Terms
-
Animals
Apoptosis
Body Weight
Cell Cycle
Gene Expression
Heart*
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Hypertension
Hypertension, Pulmonary
Hypertrophy, Right Ventricular
Male
Microarray Analysis*
Monocrotaline
Myocytes, Smooth Muscle
Natriuretic Peptide, Brain
Nitric Oxide Synthase
Rats*
RNA
Simvastatin*
Ventricular Pressure
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Monocrotaline
Natriuretic Peptide, Brain
Nitric Oxide Synthase
RNA
Simvastatin
Figure
Reference
-
1. Humbert M, Lau EM, Montani D, Jais X, Sitbon O, Simonneau G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation. 2014; 130:2189–2208.
Article2. Chen IC, Tan MS, Wu BN, Chai CY, Yeh JL, Chou SH, et al. Statins ameliorate pulmonary hypertension secondary to left ventricular dysfunction through the Rho-kinase pathway and NADPH oxidase. Pediatr Pulmonol. 2017; 52:443–457.
Article3. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016; 37:67–119.4. Lim KA, Kim KC, Cho MS, Lee BE, Kim HS, Hong YM. Gene expression of endothelin-1 and endothelin receptor a on monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2010; 40:459–464.
Article5. Koo HS, Kim KC, Hong YM. Gene expressions of nitric oxide synthase and matrix metalloproteinase-2 in monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2011; 41:83–90.
Article6. Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012; 186:261–272.
Article7. Foris V, Kovacs G, Tscherner M, Olschewski A, Olschewski H. Biomarkers in pulmonary hypertension: what do we know? Chest. 2013; 144:274–283.8. Wilson DW, Segall HJ, Pan LC, Lame MW, Estep JE, Morin D. Mechanisms and pathology of monocrotaline pulmonary toxicity. Crit Rev Toxicol. 1992; 22:307–325.
Article9. Lee YH, Kim KC, Cho MS, Hong YM. Changes of pulmonary pathology and gene expressions after simvastatin treatment in the monocrotaline-induced pulmonary hypertension rat model. Korean Circ J. 2011; 41:518–527.
Article10. Wong MJ, Kantores C, Ivanovska J, Jain A, Jankov RP. Simvastatin prevents and reverses chronic pulmonary hypertension in newborn rats via pleiotropic inhibition of RhoA signaling. Am J Physiol Lung Cell Mol Physiol. 2016; 311:L985–L999.
Article11. Schroll S, Lange TJ, Arzt M, Sebah D, Nowrotek A, Lehmann H, et al. Effects of simvastatin on pulmonary fibrosis, pulmonary hypertension and exercise capacity in bleomycin-treated rats. Acta Physiol (Oxf). 2013; 208:191–201.
Article12. Anand V, Garg S, Duval S, Thenappan T. A systematic review and meta-analysis of trials using statins in pulmonary arterial hypertension. Pulm Circ. 2016; 6:295–301.
Article13. Menon S, Fessel J, West J. Microarray studies in pulmonary arterial hypertension. Int J Clin Pract Suppl. 2011; (169):19–28.
Article14. Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res. 2014; 115:176–188.15. Buermans HP, Redout EM, Schiel AE, Musters RJ, Zuidwijk M, Eijk PP, et al. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics. 2005; 21:314–323.
Article16. Liu WH, Xu XH, Luo Q, Zhang HL, Wang Y, Xi QY, et al. Inhibition of the RhoA/Rho-associated, coiled-coil-containing protein kinase-1 pathway is involved in the therapeutic effects of simvastatin on pulmonary arterial hypertension. Clin Exp Hypertens. 2018; 40:224–230.
Article17. Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015; 21:596–608.
Article18. Laumanns IP, Fink L, Wilhelm J, Wolff JC, Mitnacht-Kraus R, Graef-Hoechst S, et al. The noncanonical WNT pathway is operative in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2009; 40:683–691.
Article19. Varshney R, Ali Q, Wu C, Sun Z. Monocrotaline-induced pulmonary hypertension involves downregulation of antiaging protein klotho and eNOS activity. Hypertension. 2016; 68:1255–1263.
Article20. Murata T, Kinoshita K, Hori M, Kuwahara M, Tsubone H, Karaki H, et al. Statin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2005; 25:2335–2342.
Article21. Jaitovich A, Jourd'heuil D. A Brief overview of nitric oxide and reactive oxygen species signaling in hypoxia-induced pulmonary hypertension. Adv Exp Med Biol. 2017; 967:71–81.
Article22. Klinger JR, Kadowitz PJ. The nitric oxide pathway in pulmonary vascular disease. Am J Cardiol. 2017; 120(8S):S71–S79.
Article23. Isaacson TC, Hampl V, Weir EK, Nelson DP, Archer SL. Increased endothelium-derived NO in hypertensive pulmonary circulation of chronically hypoxic rats. J Appl Physiol (1985). 1994; 76:933–940.
Article24. Tyler RC, Muramatsu M, Abman SH, Stelzner TJ, Rodman DM, Bloch KD, et al. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol. 1999; 276(2 Pt 1):L297–L303.25. Dupont LL, Glynos C, Bracke KR, Brouckaert P, Brusselle GG. Role of the nitric oxide-soluble guanylyl cyclase pathway in obstructive airway diseases. Pulm Pharmacol Ther. 2014; 29:1–6.
Article26. Nagaya N, Nishikimi T, Okano Y, Uematsu M, Satoh T, Kyotani S, et al. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol. 1998; 31:202–208.
Article27. Ten Kate CA, Tibboel D, Kraemer US. B-type natriuretic peptide as a parameter for pulmonary hypertension in children: a systematic review. Eur J Pediatr. 2015; 174:1267–1275.
Article28. Lammers AE, Apitz C, Zartner P, Hager A, Dubowy KO, Hansmann G. Diagnostics, monitoring and outpatient care in children with suspected pulmonary hypertension/paediatric pulmonary hypertensive vascular disease. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart. 2016; 102:Suppl 2. ii1–ii13.
Article29. Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013; 62:25 Suppl. D22–D33.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microarray Analysis in Spontaneously Hypertensive Rat Heart after Losartan Treatment
- Changes of Pulmonary Pathology and Gene Expressions After Simvastatin Treatment in the Monocrotaline-Induced Pulmonary Hypertension Rat Model
- Effect of ezetimibe and simvastatin combination in Korean hypercholesterolemic patients
- Treatment of Pulmonary Hypertensive Crisis Using ECMO: A Case Report
- The Protective Effect of Simvastatin on Monocrotaline-Induced Pulmonary Hypertension in Rats