J Korean Neurosurg Soc.
2016 Nov;59(6):551-558. 10.3340/jkns.2016.59.6.551.
Metallothinein 1E Enhances Glioma Invasion through Modulation Matrix Metalloproteinases-2 and 9 in U87MG Mouse Brain Tumor Model
- Affiliations
-
- 1Department of Neurosurgery, Chonnam National University Research Institute of Medical Sciences, Chonnam National University Medical School and Hwasun Hospital, Hwasun, Korea. sjung@jnu.ac.kr
- 2Brain Tumor Clinic and Gamma Knife Center and Brain Tumor Research Laboratory, and Chonnam National University Research Institute of Medical Sciences, Chonnam National University Medical School and Hwasun Hospital, Hwasun, Korea.
- KMID: 2417335
- DOI: http://doi.org/10.3340/jkns.2016.59.6.551
Abstract
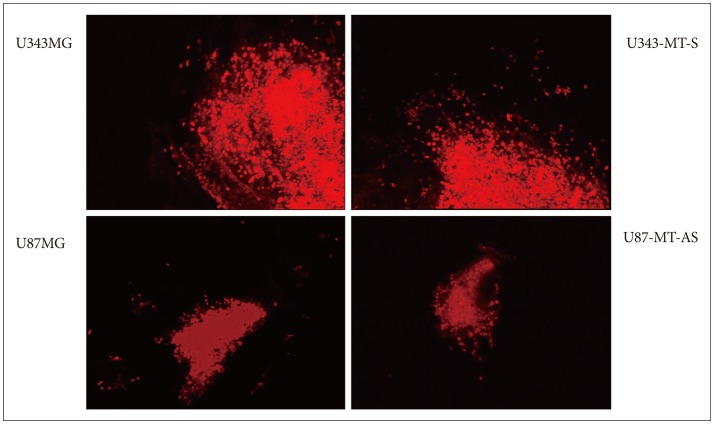
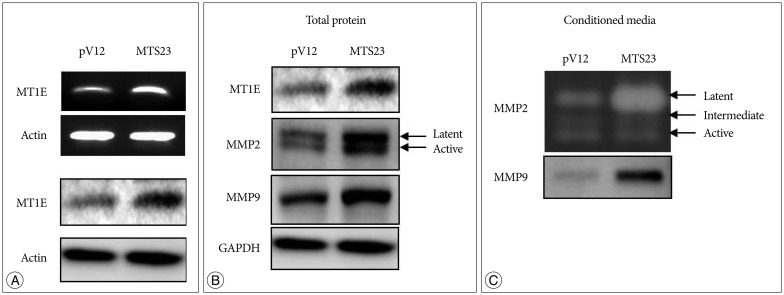
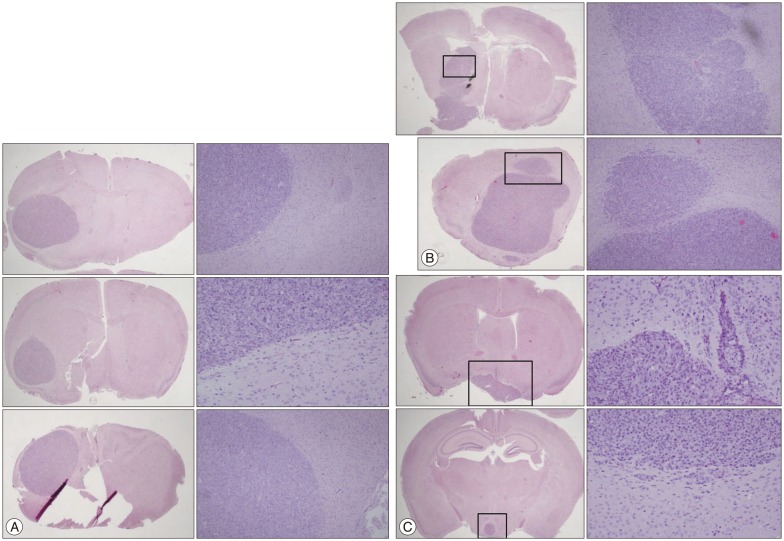
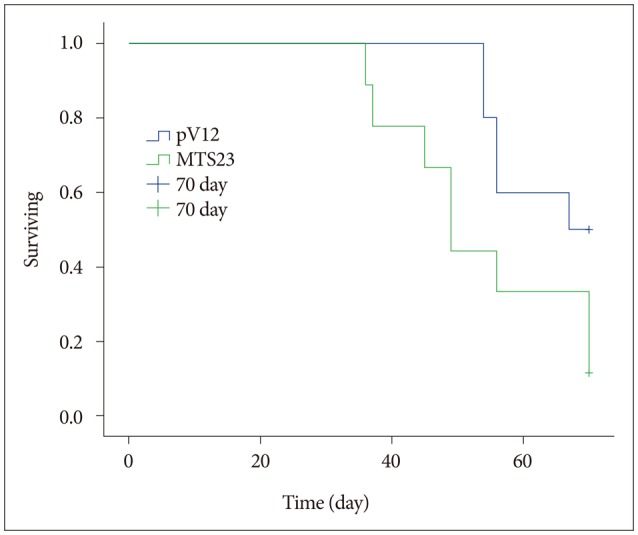
- Malignant glioma cells invading surrounding normal brain are inoperable and resistant to radio- and chemotherapy, and eventually lead to tumor regrowth. Identification of genes related to motility is important for understanding the molecular biological behavior of invasive gliomas. According to our previous studies, Metallothionein 1E (MT1E) was identified to enhance migration of human malignant glioma cells. The purpose of this study was to confirm that MT1E could modulate glioma invasion in vivo. Firstly we established 2 cell lines; MTS23, overexpressed by MT1E complementary DNA construct and pV12 as control. The expression of matrix metalloproteinases (MMP)-2, -9 and a disintegrin and metalloproteinase 17 were increased in MTS23 compared with pV12. Furthermore it was confirmed that MT1E could modulate MMPs secretion and translocation of NFkB p50 and B-cell lymphoma-3 through small interfering ribonucleic acid knocked U87MG cells. Then MTS23 and pV12 were injected into intracranial region of 5 week old male nude mouse. After 4 weeks, for brain tissues of these two groups, histological analysis, and immunohistochemical stain of MMP-2, 9 and Nestin were performed. As results, the group injected with MTS23 showed irregular margin and tumor cells infiltrating the surrounding normal brain, while that of pV12 (control) had round and clear margin. And regrowth of tumor cells in MTS23 group was observed in another site apart from tumor cell inoculation. MT1E could enhance tumor proliferation and invasion of malignant glioma through regulation of activation and expression of MMPs.
Keyword
MeSH Terms
Figure
Reference
-
1. Agrawal A, Romero-Perez D, Jacobsen JA, Villarreal FJ, Cohen SM. Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem. 2008; 3:812–820. PMID: 18181119.
Article2. Andela VB, Gordon AH, Zotalis G, Rosier RN, Goater JJ, Lewis GD, et al. NFkappaB : a pivotal transcription factor in prostate cancer metastasis to bone. Clin Orthop Relat Res. 2003; (415 Suppl):S75–S85. PMID: 14600595.3. Arriaga JM, Levy EM, Bravo AI, Bayo SM, Amat M, Aris M, et al. Metallothionein expression in colorectal cancer : relevance of different isoforms for tumor progression and patient survival. Hum Pathol. 2012; 43:197–208. PMID: 21820154.
Article4. Chen X, Chen L, Chen J, Hu W, Gao H, Xie B, et al. ADAM17 promotes U87 glioblastoma stem cell migration and invasion. Brain Res. 2013; 1538:151–158. PMID: 23470260.
Article5. Escárcega RO, Fuentes-Alexandro S, García-Carrasco M, Gatica A, Zamora A. The transcription factor nuclear factor-kappa B and cancer. Clin Oncol(R Coll Radiol). 2007; 19:154–161. PMID: 17355113.
Article6. Giricz O, Calvo V, Peterson EA, Abouzeid CM, Kenny PA. TACE-dependent TGFα shedding drives triple-negative breast cancer cell invasion. Int J Cancer. 2013; 133:2587–2595. PMID: 23729230.
Article7. Hiura T, Khalid H, Yamashita H, Tokunaga Y, Yasunaga A, Shibata S. Immunohistochemical analysis of metallothionein in astrocytic tumors in relation to tumor grade, proliferative potential, and survival. Cancer. 1998; 83:2361–2369. PMID: 9840536.
Article8. Huang ST, Yang RC, Wu HT, Wang CN, Pang JH. Zinc-chelation contributes to the anti-angiogenic effect of ellagic acid on inhibiting MMP-2 activity, cell migration and tube formation. PLoS One. 2011; 6:e18986. PMID: 21573219.
Article9. Jin R, Bay BH, Chow VT, Tan PH. Metallothionein 1F mRNA expression correlates with histological grade in breast carcinoma. Breast Cancer Res Treat. 2001; 66:265–272. PMID: 11510698.
Article10. Jung S, Ackerley C, Ivanchuk S, Mondal S, Becker LE, Rutka JT. Tracking the invasiveness of human astrocytoma cells by using green fluorescent protein in an organotypical brain slice model. J Neurosurg. 2001; 94:80–89. PMID: 11147903.
Article11. Jung S, Hinek A, Tsugu A, Hubbard SL, Ackerley C, Becker LE, et al. Astrocytoma cell interaction with elastin substrates : implications for astrocytoma invasive potential. Glia. 1999; 25:179–189. PMID: 9890632.
Article12. Jung TY, Jung S, Ryu HH, Jeong YI, Jin YH, Jin SG, et al. Role of galectin-1 in migration and invasion of human glioblastoma multiforme cell lines. J Neurosurg. 2008; 109:273–284. PMID: 18671640.
Article13. Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer : from innocent bystander to major culprit. Nat Rev Cancer. 2002; 2:301–310. PMID: 12001991.
Article14. Kim JE, Paek SH, Kim DG, Chung HT, Kim YY, Jung HW. The combined effect of gamma knife irradiation and p53 gene transfection in human malignant glioma cell lines. J Korean Neurosurg Soc. 2005; 37:48–53.15. Kim YS, Jeong YI, Jin SG, Pei J, Wen M, Kim IY, et al. Release of tissue inhibitor of metalloproteinase-2 from alginate microcapsule encapsulating genetically engineered cells. Int J Nanomedicine. 2013; 8:4351–4359. PMID: 24231999.16. Lokeshwar BL, Selzer MG, Block NL, Gunja-Smith Z. Secretion of matrix metalloproteinases and their inhibitors (tissue inhibitor of metalloproteinases) by human prostate in explant cultures: reduced tissue inhibitor of metalloproteinase secretion by malignant tissues. Cancer Res. 1993; 53:4493–4498. PMID: 7691397.17. Lv X, Li Y, Qian M, Ma C, Jing H, Wen Z, et al. ADAM17 silencing suppresses the migration and invasion of non-small cell lung cancer. Mol Med Rep. 2014; 9:1935–1940. PMID: 24626788.
Article18. Maret W, Jacob C, Vallee BL, Fischer EH. Inhibitory sites in enzymes : zinc removal and reactivation by thionein. Proc Natl Acad Sci U S A. 1999; 96:1936–1940. PMID: 10051573.19. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006; 69:562–573. PMID: 16405877.
Article20. Nakajima M, Chop AM. Tumor invasion and extracellular matrix degradative enzymes : regulation of activity by organ factors. Semin Cancer Biol. 1991; 2:115–127. PMID: 1655116.21. Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011; 12:695–708. PMID: 21772278.
Article22. Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004; 4:617–629. PMID: 15286728.
Article23. Roesijadi G. Metal transfer as a mechanism for metallothionein-mediated metal detoxification. Cell Mol Biol (Noisy-le-grand). 2000; 46:393–405. PMID: 10774928.24. Ryu HH, Jung S, Jung TY, Moon KS, Kim IY, Jeong YI, et al. Role of metallothionein 1E in the migration and invasion of human glioma cell lines. Int J Oncol. 2012; 41:1305–1313. PMID: 22843066.
Article25. Satoh M, Cherian MG, Imura N, Shimizu H. Modulation of resistance to anticancer drugs by inhibition of metallothionein synthesis. Cancer Res. 1994; 54:5255–5257. PMID: 7923149.26. Werynska B, Pula B, Muszczynska-Bernhard B, Piotrowska A, Jethon A, Podhorska-Okolow M, et al. Correlation between expression of metallothionein and expression of Ki-67 and MCM-2 proliferation markers in non-small cell lung cancer. Anticancer Res. 2011; 31:2833–2839. PMID: 21868526.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proteomic Analysis between U87MG and U343MG-A Cell Lines: Searching for Candidate Proteins for Glioma Invasion
- Expression of Matrix Metalloproteinases and Its Inhibitor in Gastric Adenocarcinoma
- Inhibitory Effects of Toxoplasma Antigen on Proliferation and Invasion of Human Glioma Cells
- The Effect of Chemoradiotherapy with SRC Tyrosine Kinase Inhibitor, PP2 and Temozolomide on Malignant Glioma Cells In Vitro and In Vivo
- High Expression of KIFC1 in Glioma Correlates with Poor Prognosis