Int J Stem Cells.
2018 Jun;11(1):26-38. 10.15283/ijsc17015.
CD73, CD90, CD105 and Cadherin-11 RT-PCR Screening for Mesenchymal Stem Cells from Cryopreserved Human Cord Tissue
- Affiliations
-
- 1Processing Laboratory, StemCyte International Cord Blood Therapeutic Company, Baldwin Park, CA, USA. mchen@stemcyte.com
- 2Department of Medicine, University of California – Los Angeles, Los Angeles, CA, USA.
- 3Department of Medicine, Veterans Affair, Greater Los Angeles Healthcare System, Los Angeles, CA, USA.
- 4Tissue Laboratory, StemCyte, New Taipei City Linkou District, Taiwan.
- 5University of Bologna, Sant' Orsola-Malpighi Hospital, Bologna, Italy.
- KMID: 2413498
- DOI: http://doi.org/10.15283/ijsc17015
Abstract
- BACKGROUND AND OBJECTIVES
Mesenchymal stem cells (MSCs) are self-renewing, non-specialized cells used clinically in tissue regeneration and sourced from bone marrow, peripheral blood, umbilical cord blood and umbilical cord tissue (UCT). To demonstrate an alternative method for MSC detection, cryopreserved UCT and expanded MSC were screened for MSC markers CD73, CD90, CD105 and CDH-11 by RT-PCR.
METHODS AND RESULTS
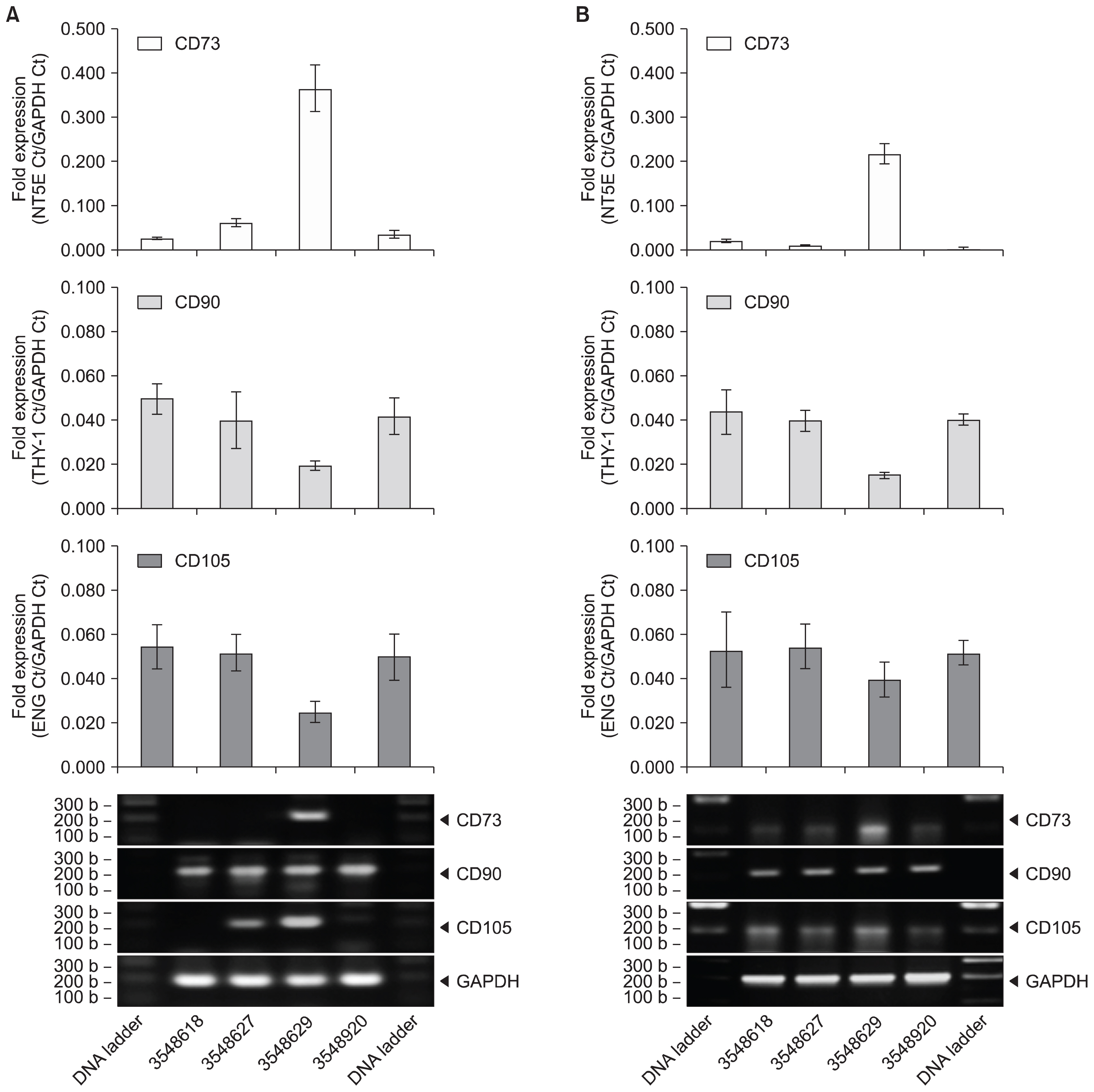
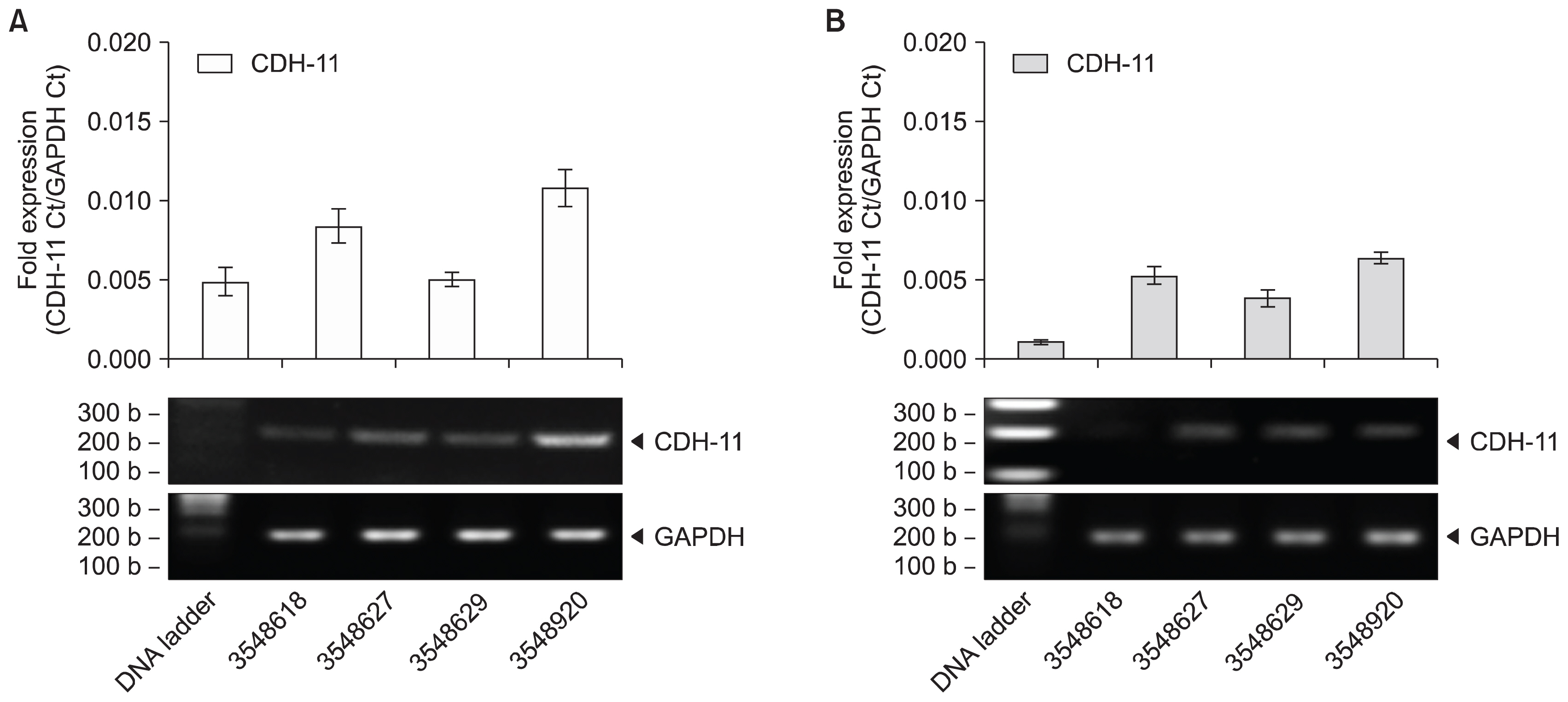
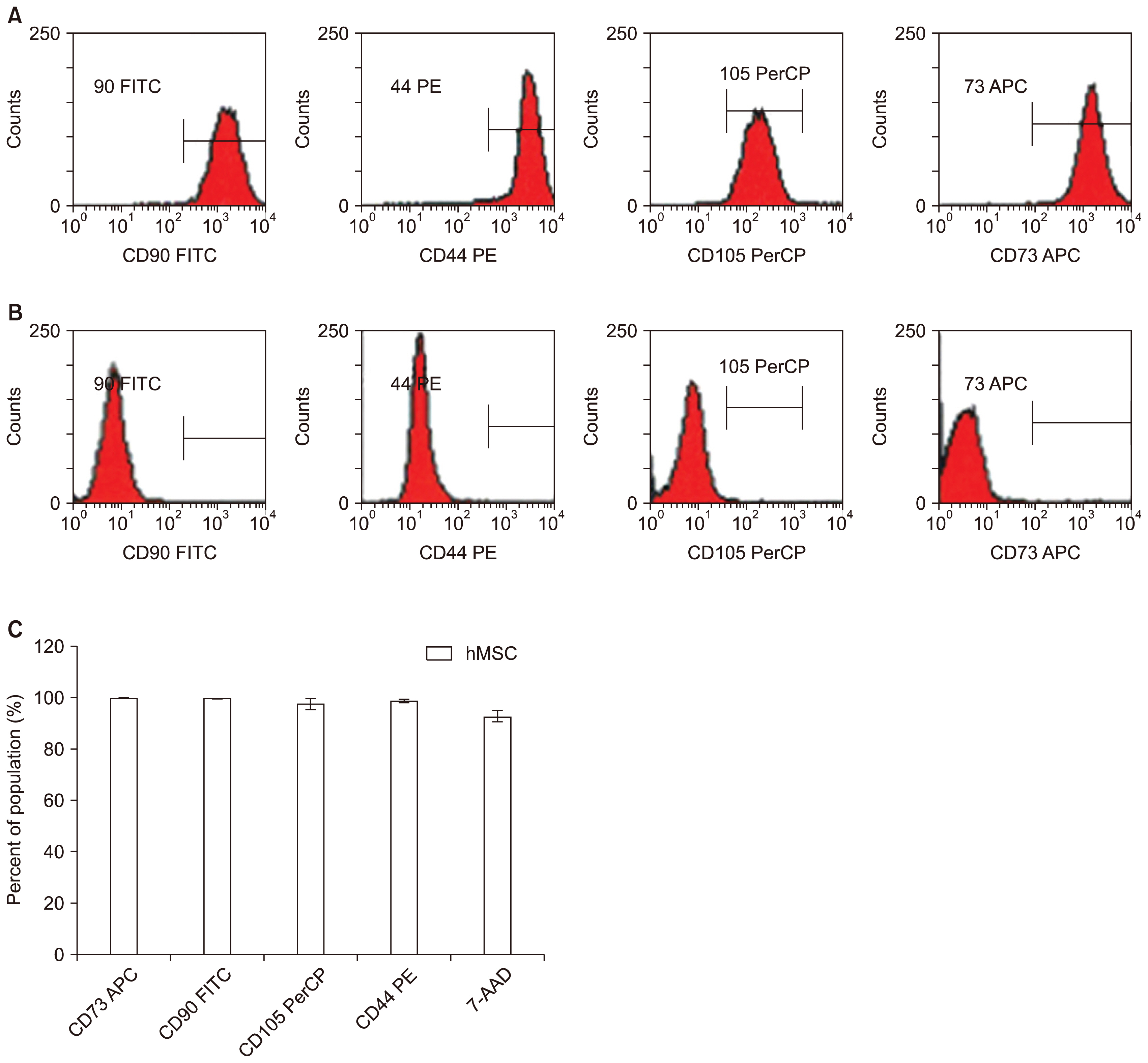
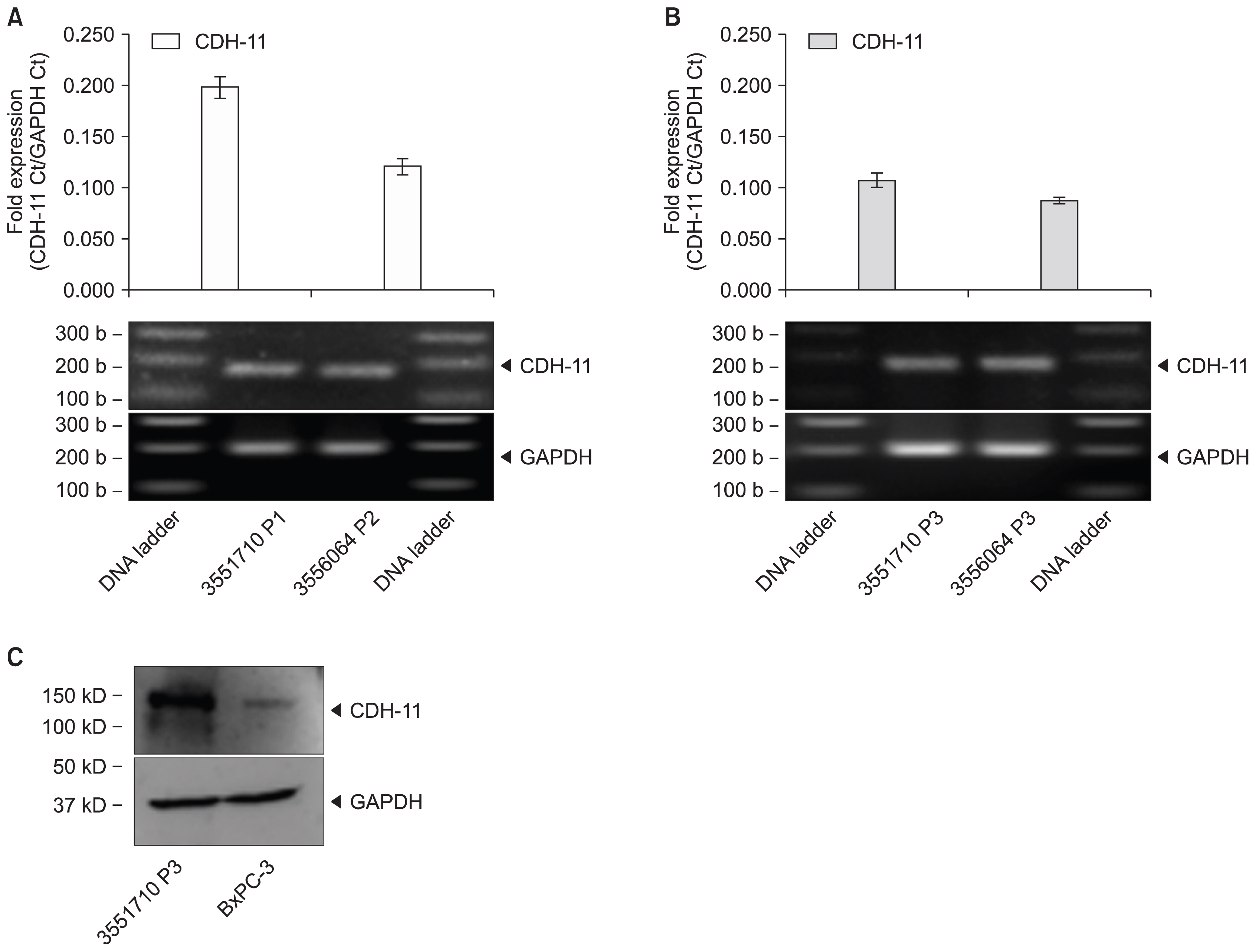
Human UCT were washed, sectioned, cryopreserved with 10% DMSO and stored in the vapor phase of liquid nitrogen. Fresh and frozen UCT samples were expanded for MSC. UCT and MSC were processed for RNA and screened for CD73, CD90, CD105 and CDH-11 mRNA by RT-PCR. CD73, CD90 and CD105 were detected by flow cytometry and CDH-11 was detected by Western blotting. Short and long-term frozen UCT shows a loss of mRNA expression for CD73 at 33.2±34.0%, CD90 at 6.2±8.2%, CD105 at 17.7±21.6% and CDH-11 at 30.1±26.7% but was not statistically significant to indicate the deterioration. Expanded MSCs from fresh UCT expressed 0.09±0.07-fold CD73, 0.17±0.11-fold CD90, 0.04±0.06-fold CD105 and 0.14±0.08-fold CDH-11. Expanded MSCs from frozen UCTs expressed 0.09±0.06-fold CD73, 0.13±0.06-fold CD90, 0.04±0.05-fold CD105 and 0.11±0.06-fold CDH-11 and confirmed by flow cytometry and Western blotting.
CONCLUSION
CD73, CD90, CD105 and CDH-11 were detected by RT-PCR in cryopreserved UCT and MSC expansion. CDH-11 appears as a useful single target MSC marker for quick screening.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases
Hussein Baharlooi, Maryam Azimi, Zahra Salehi, Maryam Izad
Int J Stem Cells. 2019;13(1):13-23. doi: 10.15283/ijsc19108.Effect of Substrate Topography and Chemistry on Human Mesenchymal Stem Cell Markers: A Transcriptome Study
Bo Zhang, Naresh Kasoju, Qiongfang Li, Jinmin Ma, Aidong Yang, Zhanfeng Cui, Hui Wang, Hua Ye
Int J Stem Cells. 2019;12(1):84-94. doi: 10.15283/ijsc18102.
Reference
-
References
1. Weiner LP. Definitions and criteria for stem cells. Methods Mol Biol. 2008; 438:3–8. DOI: 10.1007/978-1-59745-133-8_1. PMID: 18369744.
Article2. Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015; 17:11–22. DOI: 10.1016/j.stem.2015.06.007. PMID: 26140604.
Article3. Thomas ED. Bone marrow transplantation: a review. Semin Hematol. 1999; 36(4 Suppl 7):95–103. PMID: 10595758.4. Reinke S, Dienelt A, Blankenstein A, Duda GN, Geissler S. Qualifying stem cell sources: how to overcome potential pitfalls in regenerative medicine? J Tissue Eng Regen Med. 2016; 10:3–10. DOI: 10.1002/term.1923.
Article5. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 6:230–247. DOI: 10.1097/00007890-196803000-00009. PMID: 5654088.6. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147. DOI: 10.1126/science.284.5411.143. PMID: 10102814.
Article7. Jadalannagari S, Aljitawi OS. Ectodermal differentiation of Wharton’s Jelly mesenchymal stem cells for tissue engineering and regenerative medicine applications. Tissue Eng Part B Rev. 2015; 21:314–322. DOI: 10.1089/ten.teb.2014.0404.
Article8. Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012; 8:375–392. DOI: 10.1007/s12015-011-9312-0.
Article9. Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014; 6:195–202. DOI: 10.4252/wjsc.v6.i2.195. PMID: 24772246. PMCID: 3999777.
Article10. Fazzina R, Mariotti A, Procoli A, Fioravanti D, Iudicone P, Scambia G, Pierelli L, Bonanno G. A new standardized clinical-grade protocol for banking human umbilical cord tissue cells. Transfusion. 2015; 55:2864–2873. DOI: 10.1111/trf.13277. PMID: 26354088.
Article11. Van Pham P, Phan NK. Production of good manufacturing practice-grade human umbilical cord blood-derived mesenchymal stem cells for therapeutic use. Methods Mol Biol. 2015; 1283:73–85. DOI: 10.1007/7651_2014_125.
Article12. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. DOI: 10.1080/14653240600855905. PMID: 16923606.
Article13. Maleki M, Ghanbarvand F, Reza Behvarz M, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014; 7:118–126. DOI: 10.15283/ijsc.2014.7.2.118. PMID: 25473449. PMCID: 4249894.
Article14. Chatzistamatiou TK, Papassavas AC, Michalopoulos E, Gamaloutsos C, Mallis P, Gontika I, Panagouli E, Koussoulakos SL, Stavropoulos-Giokas C. Optimizing isolation culture and freezing methods to preserve Wharton’s jelly’s mesenchymal stem cell (MSC) properties: an MSC banking protocol validation for the Hellenic Cord Blood Bank. Transfusion. 2014; 54:3108–3120. DOI: 10.1111/trf.12743. PMID: 24894363.
Article15. Simonneau L, Kitagawa M, Suzuki S, Thiery JP. Cadherin 11 expression marks the mesenchymal phenotype: towards new functions for cadherins? Cell Adhes Commun. 1995; 3:115–130. DOI: 10.3109/15419069509081281. PMID: 7583005.
Article16. Christopoulos PF, Bournia VK, Panopoulos S, Vaiopoulos A, Koutsilieris M, Sfikakis PP. Increased messenger RNA levels of the mesenchymal cadherin-11 in the peripheral blood of systemic sclerosis patients correlate with diffuse skin involvement. Clin Exp Rheumatol. 2015; 33(4 Suppl 91):S36–S39. PMID: 26121083.17. MacCalman CD, Furth EE, Omigbodun A, Bronner M, Coutifaris C, Strauss JF 3rd. Regulated expression of cadherin-11 in human epithelial cells: a role for cadherin-11 in trophoblast-endometrium interactions? Dev Dyn. 1996; 206:201–211. DOI: 10.1002/(SICI)1097-0177(199606)206:2<201::AID-AJA9>3.0.CO;2-M. PMID: 8725287.
Article18. Shi S, Jia S, Liu J, Chen G. Accelerated regeneration of skin injury by co-transplantation of mesenchymal stem cells from Wharton’s jelly of the human umbilical cord mixed with microparticles. Cell Biochem Biophys. 2015; 71:951–956. DOI: 10.1007/s12013-014-0292-y.
Article19. Roy S, Arora S, Kumari P, Ta M. A simple and serum-free protocol for cryopreservation of human umbilical cord as source of Wharton’s jelly mesenchymal stem cells. Cryobiology. 2014; 68:467–472. DOI: 10.1016/j.cryobiol.2014.03.010. PMID: 24704519.
Article20. Mechiche Alami S, Velard F, Draux F, Siu Paredes F, Josse J, Lemaire F, Gangloff SC, Graesslin O, Laurent-Maquin D, Kerdjoudj H. Gene screening of Wharton’s jelly derived stem cells. Biomed Mater Eng. 2014; 24(1 Suppl):53–61. PMID: 24928918.
Article21. Alimperti S, Andreadis ST. CDH2 and CDH11 act as regulators of stem cell fate decisions. Stem Cell Res. 2015; 14:270–282. DOI: 10.1016/j.scr.2015.02.002. PMID: 25771201. PMCID: 4439315.
Article22. Kii I, Amizuka N, Shimomura J, Saga Y, Kudo A. Cell-cell interaction mediated by cadherin-11 directly regulates the differentiation of mesenchymal cells into the cells of the osteo-lineage and the chondro-lineage. J Bone Miner Res. 2004; 19:1840–1849. DOI: 10.1359/JBMR.040812. PMID: 15476585.
Article23. Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014; 32:1408–1419. DOI: 10.1002/stem.1681. PMID: 24578244.
Article24. Boscher C, Mège RM. Cadherin-11 interacts with the FGF receptor and induces neurite outgrowth through associated downstream signalling. Cell Signal. 2008; 20:1061–1072. DOI: 10.1016/j.cellsig.2008.01.008. PMID: 18302981.
Article25. Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev. 2013; 9:226–240. DOI: 10.1007/s12015-012-9418-z.
Article26. Rossignoli F, Caselli A, Grisendi G, Piccinno S, Burns JS, Murgia A, Veronesi E, Loschi P, Masini C, Conte P, Paolucci P, Horwiz EM, Dominici M. Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. Biomed Res Int. 2013; DOI: 10.1155/2013/901821. PMID: 23607099. PMCID: 3626323.
Article27. Pirjali T, Azarpira N, Ayatollahi M, Aghdaie MH, Geramizadeh B, Talai T. Isolation and characterization of human mesenchymal stem cells derived from human umbilical cord Wharton’s Jelly and amniotic membrane. Int J Organ Transplant Med. 2013; 4:111–116.28. Harris DT. Umbilical cord tissue mesenchymal stem cells: characterization and clinical applications. Curr Stem Cell Res Ther. 2013; 8:394–399. DOI: 10.2174/1574888X11308050006. PMID: 23895058.
Article29. Hendijani F, Sadeghi-Aliabadi H, Haghjooy Javanmard S. Comparison of human mesenchymal stem cells isolated by explant culture method from entire umbilical cord and Wharton’s jelly matrix. Cell Tissue Bank. 2014; 15:555–565. DOI: 10.1007/s10561-014-9425-1. PMID: 24532125.
Article30. Han YF, Tao R, Sun TJ, Chai JK, Xu G, Liu J. Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods. Cytotechnology. 2013; 65:819–827. DOI: 10.1007/s10616-012-9528-0. PMID: 23306781. PMCID: 3967601.
Article31. Tsagias N, Koliakos I, Karagiannis V, Eleftheriadou M, Koliakos GG. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus Med. 2011; 21:253–261. DOI: 10.1111/j.1365-3148.2011.01076.x. PMID: 21623971.
Article32. Ding Y, Yang H, Feng JB, Qiu Y, Li DS, Zeng Y. Human umbilical cord-derived MSC culture: the replacement of animal sera with human cord blood plasma. In Vitro Cell Dev Biol Anim. 2013; 49:771–777. DOI: 10.1007/s11626-013-9663-8. PMID: 24043577.
Article33. Corotchi MC, Popa MA, Remes A, Sima LE, Gussi I, Lupu Plesu M. Isolation method and xeno-free culture conditions influence multipotent differentiation capacity of human Wharton’s jelly-derived mesenchymal stem cells. Stem Cell Res Ther. 2013; 4:81. DOI: 10.1186/scrt232.
Article34. Kim SM, Moon SH, Lee Y, Kim GJ, Chung HM, Choi YS. Alternative xeno-free biomaterials derived from human umbilical cord for the self-renewal ex-vivo expansion of mesenchymal stem cells. Stem Cells Dev. 2013; 22:3025–3038. DOI: 10.1089/scd.2013.0067. PMID: 23786292.
Article35. Pereira T, Ivanova G, Caseiro AR, Barbosa P, Bártolo PJ, Santos JD, Luís AL, Maurício AC. MSCs conditioned media and umbilical cord blood plasma metabolomics and composition. PLoS One. 2014; 9:e113769. DOI: 10.1371/journal.pone.0113769. PMID: 25423186. PMCID: 4244191.
Article36. Pham PV, Vu NB, Pham VM, Truong NH, Pham TL, Dang LT, Nguyen TT, Bui AN, Phan NK. Good manufacturing practice-compliant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. J Transl Med. 2014; 12:56. DOI: 10.1186/1479-5876-12-56. PMID: 24565047. PMCID: 3939935.
Article37. Kinzebach S, Bieback K. Expansion of Mesenchymal Stem/Stromal cells under xenogenic-free culture conditions. Adv Biochem Eng Biotechnol. 2013; 129:33–57.
Article38. Swamynathan P, Venugopal P, Kannan S, Thej C, Kolkundar U, Bhagwat S, Ta M, Majumdar AS, Balasubramanian S. Are serum-free and xeno-free culture conditions ideal for large scale clinical grade expansion of Wharton’s jelly derived mesenchymal stem cells? A comparative study. Stem Cell Res Ther. 2014; 5:88. DOI: 10.1186/scrt477.
Article39. Chen G, Yue A, Ruan Z, Yin Y, Wang R, Ren Y, Zhu L. Human umbilical cord-derived mesenchymal stem cells do not undergo malignant transformation during long-term culturing in serum-free medium. PLoS One. 2014; 9:e98565. DOI: 10.1371/journal.pone.0098565. PMID: 24887492. PMCID: 4041760.
Article40. Yang LM, Liu Y, Zhao J, Hao LM, Huang KX, Jiang WH. Characterization of human umbilical cord mesenchymal stem cells following tissue mass culture. Cell Mol Biol (Noisy-le-grand). 2014; 60:12–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolation and Characterization of Human Chorionic Membranes Mesenchymal Stem Cells and Their Neural Differentiation
- Mesenchymal Stem Cell Therapy in Pulmonary Disease
- Isolation and Characterization of Human Suture Mesenchymal Stem Cells In Vitro
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Long Term Exposure to Myrtucommulone-A Changes CD105 Expression and Differentiation Potential of Mesenchymal Stem Cells