J Vet Sci.
2016 Dec;17(4):453-458. 10.4142/jvs.2016.17.4.453.
Paclitaxel inhibits the hyper-activation of spleen cells by lipopolysaccharide and induces cell death
- Affiliations
-
- 1Laboratory of Veterinary Pharmacology, College of Veterinary Medicine, Jeju National University, Jeju 63243, Korea. jooh@jejunu.ac.kr
- KMID: 2412600
- DOI: http://doi.org/10.4142/jvs.2016.17.4.453
Abstract
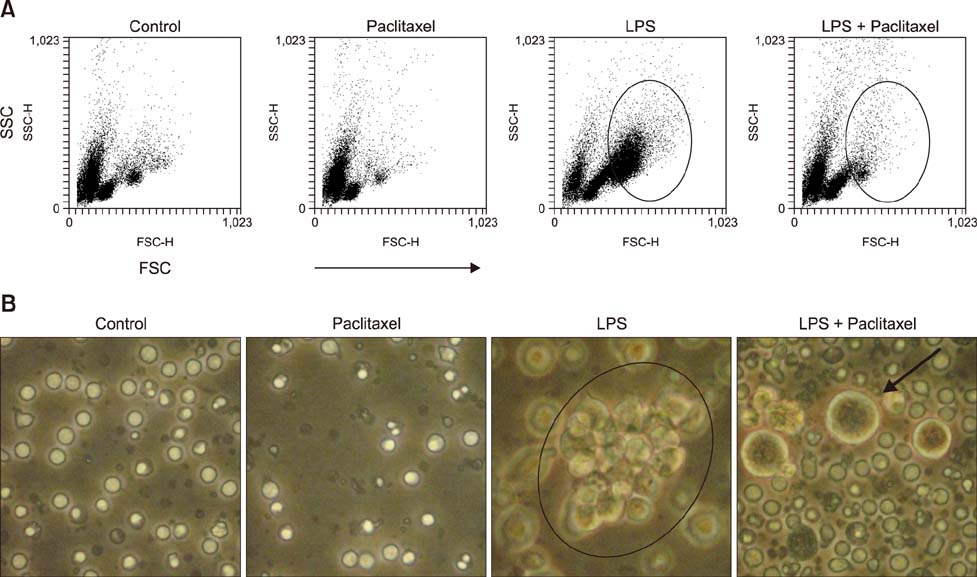
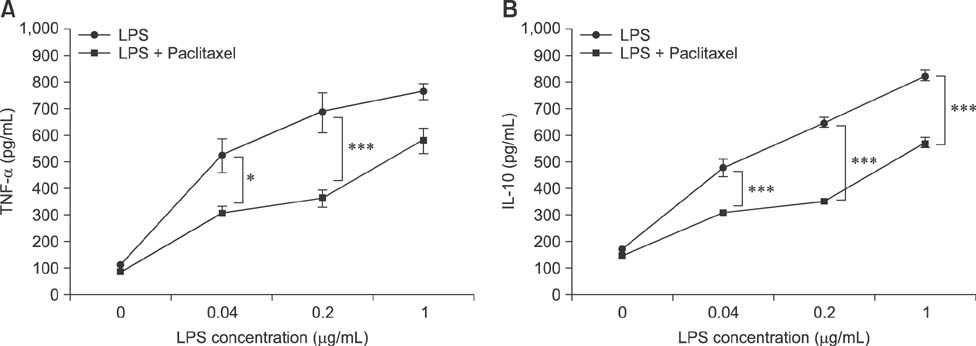
- Paclitaxel was isolated from the bark of the Pacific yew, Taxus brevifolia, and used as an anticancer agent. Paclitaxel prevents cancer cell division by inhibiting spindle fiber function, inducing cell death. A recent study demonstrated that paclitaxel binds to myeloid differentiation protein-2 of Toll-like receptor 4 and prevents the signal transduction of lipopolysaccharide (LPS). Paclitaxel converts immune cells hypo-responsive to LPS. In this study, we investigated whether paclitaxel can inhibit the phenotype and function of immune cells. To accomplish this, we used spleen cells, a major type of immune cell, LPS, a representative inflammatory agent and a mitogen for B lymphocytes. LPS profoundly increased the activation and cytokine production of spleen cells. However, paclitaxel significantly inhibited LPS-induced hyper-activation of spleen cells. Furthermore, we found that paclitaxel induced cell death of LPS-treated spleen cells. These results suggest that paclitaxel can inhibit the hyper-immune response of LPS in spleen cells via a variety of mechanisms. These findings suggest that paclitaxel can be used as a modulating agent for diseases induced by hyper-activation of B lymphocytes. Taken together, these results demonstrate that paclitaxel inhibits the function of spleen cells activated by LPS, and further induces cell death.
MeSH Terms
-
Animals
Antineoplastic Agents, Phytogenic/*pharmacology
Apoptosis/*drug effects
Cell Proliferation/*drug effects
Cytokines/*metabolism
Lipopolysaccharides/toxicity
Mice
Mice, Inbred BALB C
Mice, Inbred C57BL
Paclitaxel/*pharmacology
Spleen/*drug effects
Antineoplastic Agents, Phytogenic
Cytokines
Lipopolysaccharides
Paclitaxel
Figure
Reference
-
1. Byon YY, Kim MH, Yoo ES, Hwang KK, Jee Y, Shin T, Joo HG. Radioprotective effects of fucoidan on bone marrow cells: improvement of the cell survival and immunoreactivity. J Vet Sci. 2008; 9:359–365.
Article2. Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001; 31:2448–2457.
Article3. Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975; 72:3666–3670.
Article4. Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004; 9:1433–1449.
Article5. Guchelaar HJ, ten Napel CH, de Vries EG, Mulder NH. Clinical, toxicological and pharmaceutical aspects of the antineoplastic drug taxol: a review. Clin Oncol (R Coll Radiol). 1994; 6:40–48.
Article6. Hamann J, Fiebig H, Strauss M. Expression cloning of the early activation antigen CD69, a type II integral membrane protein with a C-type lectin domain. J Immunol. 1993; 150:4920–4927.7. Jang JY, Moon SY, Joo HG. Differential effects of fucoidans with low and high molecular weight on the viability and function of spleen cells. Food Chem Toxicol. 2014; 68:234–238.
Article8. Javeed A, Ashraf M, Riaz A, Ghafoor A, Afzal S, Mukhtar MM. Paclitaxel and immune system. Eur J Pharm Sci. 2009; 38:283–290.
Article9. Joo HG. Altered maturation of dendritic cells by taxol, an anticancer drug. J Vet Sci. 2003; 4:229–234.
Article10. Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a β-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001; 69:555–564.11. Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Mouse Toll-like receptor 4.MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem. 2000; 275:2251–2254.
Article12. Kim HJ, Kim MH, Byon YY, Park JW, Jee Y, Joo HG. Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J Vet Sci. 2007; 8:39–44.
Article13. Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008; 115:138–143.
Article14. Kim MH, Joo HG. The role of Bcl-xL and nuclear factor-κB in the effect of taxol on the viability of dendritic cells. J Vet Sci. 2009; 10:99–103.
Article15. Kohler DR, Goldspiel BR. Paclitaxel (taxol). Pharmacotherapy. 1994; 14:3–34.
Article16. Lee M, Yea SS, Jeon YJ. Paclitaxel causes mouse splenic lymphocytes to a state hyporesponsive to lipopolysaccharide stimulation. Int J Immunopharmacol. 2000; 22:615–621.
Article17. Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010; 33:153–165.
Article18. Möller G. Lipopolysaccharide as a tool to reveal autoreactive B cells. APMIS. 1988; 96:93–100.
Article19. Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008; 226:205–218.
Article20. Nehate C, Jain S, Saneja A, Khare V, Alam N, Dubey RD, Gupta PN. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014; 11:666–686.
Article21. Opal SM. The clinical relevance of endotoxin in human sepsis: a critical analysis. J Endotoxin Res. 2002; 8:473–476.
Article22. Resman N, Gradišar H, Vašl J, Keber MM, Pristovšek P, Jerala R. Taxanes inhibit human TLR4 signaling by binding to MD-2. FEBS Lett. 2008; 582:3929–3934.
Article23. Rowinsky EK, Onetto N, Canetta RM, Arbuck SG. Taxol: the first of the taxanes, an important new class of antitumor agents. Semin Oncol. 1992; 19:646–662.24. Sugrue MM, Tatton WG. Mitochondrial membrane potential in aging cells. Biol Signals Recept. 2001; 10:176–188.
Article25. Zhang D, Li Y, Liu Y, Xiang X, Dong Z. Paclitaxel ameliorates lipopolysaccharide-induced kidney injury by binding myeloid differentiation protein-2 to block Toll-like receptor 4-mediated nuclear factor-κB activation and cytokine production. J Pharmacol Exp Ther. 2013; 345:69–75.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Modulatory action of enrofloxacin in lipopolysaccharide-induced hyper-activated mouse spleen cells
- Induction of apoptosis in mouse spleen cells by Ginsenoside Rp1
- Blockade of Autophagy Aggravates Endoplasmic Reticulum Stress and Improves Paclitaxel Cytotoxicity in Human Cervical Cancer Cells
- Anti-inflammatory effects of 4,4'-diaminodiphenyl sulfone (dapsone) in lipopolysaccharide-treated spleen cells: selective inhibition of inflammation-related cytokines
- Mechanism of FHIT-Induced Apoptosis in Lung Cancer Cell Lines