J Vet Sci.
2017 Aug;18(S1):371-379. 10.4142/jvs.2017.18.S1.371.
Establishment of minimal positive-control conditions to ensure brain safety during rapid development of emergency vaccines
- Affiliations
-
- 1Department of Biomedical Laboratory Science, College of Medical Sciences, Soonchunhyang University, Asan 31538, Korea. admiral96@sch.ac.kr
- 2Department of Health Science, Konyang University, Nonsan 32992, Korea.
- 3Department of Molecular Imaging, Korea Institute of Radiology and Medical Sciences, Seoul 01812, Korea.
- 4College of Veterinary Medicine and Institute of Veterinary Science, Kangwon National University, Chuncheon 24341, Korea.
- KMID: 2412562
- DOI: http://doi.org/10.4142/jvs.2017.18.S1.371
Abstract
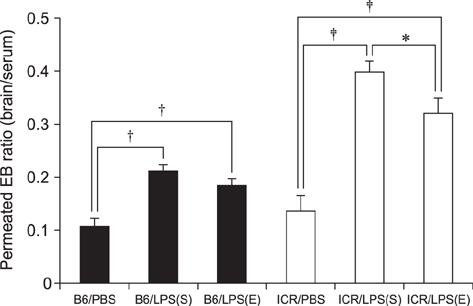
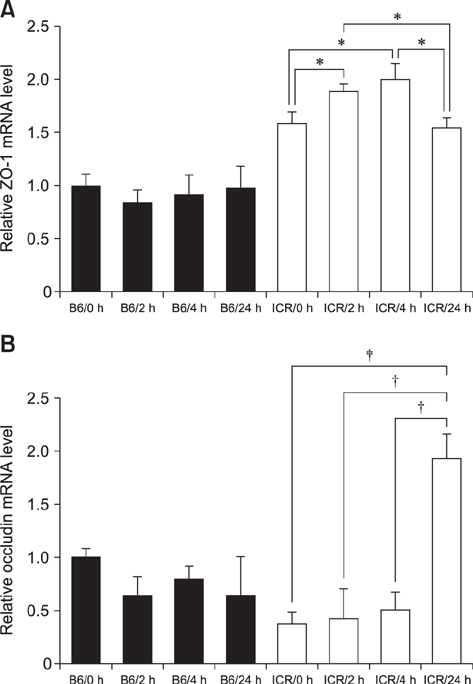
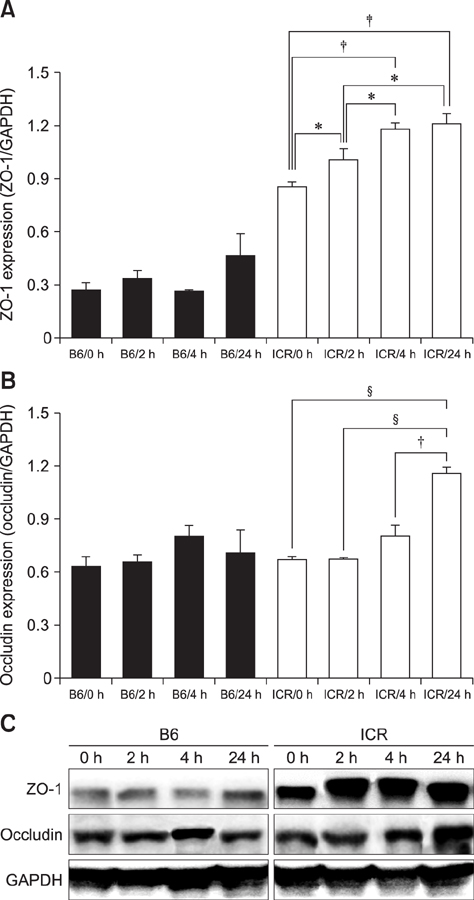
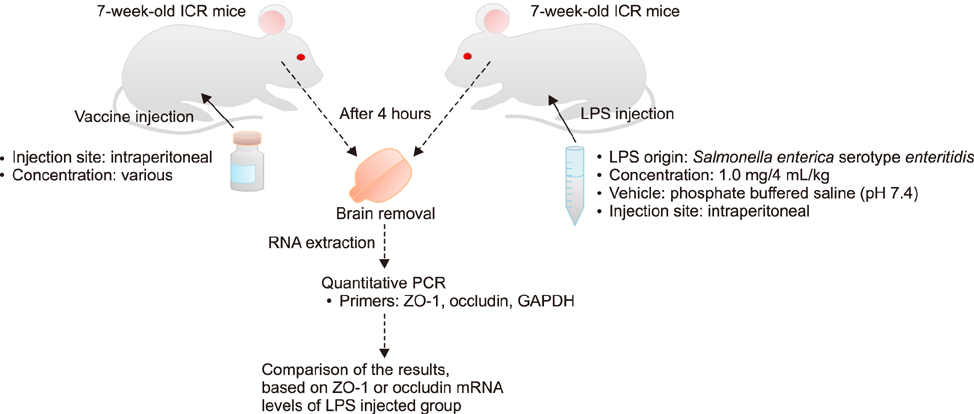
- With the increase in international human and material exchanges, contagious and infectious epidemics are occurring. One of the effective methods of epidemic inhibition is the rapid development and supply of vaccines. Considering the safety of the brain during vaccine development is very important. However, manuals for brain safety assays for new vaccines are not uniform or effective globally. Therefore, the aim of this study is to establish a positive-control protocol for an effective brain safety test to enhance rapid vaccine development. The blood-brain barrier's tight junctions provide selective defense of the brain; however, it is possible to destroy these important microstructures by administering lipopolysaccharides (LPSs), thereby artificially increasing the permeability of brain parenchyma. In this study, test conditions are established so that the degree of brain penetration or brain destruction of newly developed vaccines can be quantitatively identified. The most effective conditions were suggested by measuring time-dependent expressions of tight junction biomarkers (zonula occludens-1 [ZO-1] and occludin) in two types of mice (C57BL/6 and ICR) following exposure to two types of LPS (Salmonella and Escherichia). In the future, we hope that use of the developed positive-control protocol will help speed up the determination of brain safety of novel vaccines.
MeSH Terms
-
Animals
Biomarkers/metabolism
Blood-Brain Barrier
Brain/*drug effects
*Emergencies
Epidemics/prevention & control
Lipopolysaccharides/pharmacology
Mice
Mice, Inbred C57BL
Mice, Inbred ICR
Occludin/metabolism
*Safety
Tight Junctions/metabolism
Vaccines/*adverse effects
Zonula Occludens-1 Protein/metabolism
Biomarkers
Lipopolysaccharides
Occludin
Vaccines
Zonula Occludens-1 Protein
Figure
Reference
-
1. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004; 16:1–13.2. Banks WA, Gray AM, Erickson MA, Salameh TS, Damodarasamy M, Sheibani N, Meabon JS, Wing EE, Morofuji Y, Cook DG, Reed MJ. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation. 2015; 12:223.
Article3. Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim Biophys Acta. 2008; 1778:770–793.
Article4. Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008; 1778:588–600.
Article5. Cummins PM. Occludin: one protein, many forms. Mol Cell Biol. 2012; 32:242–250.
Article6. Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009; 9:715–720.
Article7. Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009; 16:1590–1598.
Article8. Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998; 273:29745–29753.
Article9. Fu BM. Experimental methods and transport models for drug delivery across the blood-brain barrier. Curr Pharm Biotechnol. 2012; 13:1346–1359.
Article10. Glezer I, Chernomoretz A, David S, Plante MM, Rivest S. Genes involved in the balance between neuronal survival and death during inflammation. PLoS One. 2007; 2:e310.
Article11. Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013; 93:525–569.
Article12. Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair. 2010; 3:21.
Article13. Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001; 24:719–725.
Article14. Jangula A, Murphy EJ. Lipopolysaccharide-induced blood brain barrier permeability is enhanced by alpha-synuclein expression. Neurosci Lett. 2013; 551:23–27.
Article15. Kitler ME, Gavinio P, Lavanchy D. Influenza and the work of the World Health Organization. Vaccine. 2002; 20:Suppl 2. S5–S14.
Article16. Malaowalla AM, Fong C. Toxicity of Evans blue dye in the monkey and tracing of it in the tooth pulp. Oral Surg Oral Med Oral Pathol. 1962; 15:1259–1263.
Article17. Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans Blue injection routes: intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011; 195:206–210.
Article18. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009; 22:240–273. Table of Contents.
Article19. Nicolaides C, Cueto-Felgueroso L, González MC, Juanes R. A metric of influential spreading during contagion dynamics through the air transportation network. PLoS One. 2012; 7:e40961.
Article20. Parameswaran N, Patial S. Tumor necrosis factor-a signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010; 20:87–103.21. Qin LH, Huang W, Mo XA, Chen YL, Wu XH. LPS Induces occludin dysregulation in cerebral microvascular endothelial cells via MAPK signaling and augmenting MMP-2 levels. Oxid Med Cell Longev. 2015; 2015:120641.
Article22. Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol. 2008; 6:179–192.
Article23. Tatem AJ, Rogers DJ, Hay SI. Global transport networks and infectious disease spread. Adv Parasitol. 2006; 62:293–343.
Article24. Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010; 123:2844–2852.
Article25. Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017; 60:1–12.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Verification with the utility of an established rapid assessment of brain safety for newly developed vaccines
- Adverse events and preventive measures related to COVID-19 vaccines
- New Vaccine Technology for Control of Emerging and Reemerging Infectious Diseases
- Management of vaccine safety in Korea
- Lessons from US FDA's COVID-19 Related Post-Marketing Drug Safety Monitoring and Evaluation Activities