Clin Exp Vaccine Res.
2013 Jan;2(1):40-45.
Management of vaccine safety in Korea
- Affiliations
-
- 1Division of Vaccine Preventable Disease Control and National Immunization Program, Korea Centers for Disease Control and Prevention, Cheongwon, Korea. bgr824@cdc.go.kr
Abstract
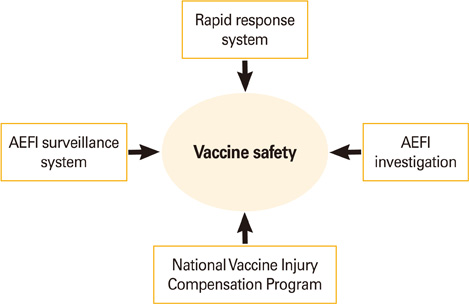
- Although vaccination is regarded as one of the most effective public health measure to prevent and control infectious diseases, no vaccine is perfectly safe. Therefore, safety management is an essential component in running National Immunization Program. Here, we review the current issues and suggest future perspectives of Korean vaccine safety management system.
MeSH Terms
Figure
Reference
-
1. Leventhal JS, Berger EM, Brauer JA, Cohen DE. Hypersensitivity reactions to vaccine constituents: a case series and review of the literature. Dermatitis. 2012. 23:102–109.2. Forsdahl BA. Reactions of Norwegian children with severe egg allergy to an egg-containing influenza A (H1N1) vaccine: a retrospective audit. BMJ Open. 2012. 2:e000186.3. Kelso JM, Jones RT, Yunginger JW. Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin. J Allergy Clin Immunol. 1993. 91:867–872.
Article4. Clements CJ, McIntyre PB. When science is not enough: a risk/benefit profile of thiomersal-containing vaccines. Expert Opin Drug Saf. 2006. 5:17–29.
Article5. Shefer A, Dales L, Nelson M, Werner B, Baron R, Jackson R. Use and safety of acellular pertussis vaccine among adult hospital staff during an outbreak of pertussis. J Infect Dis. 1995. 171:1053–1056.
Article6. Verbov J. Local skin complications of BCG vaccination. Practitioner. 1984. 228:1069–1071.7. Deloria MA, Blackwelder WC, Decker MD, et al. Association of reactions after consecutive acellular or whole-cell pertussis vaccine immunizations. Pediatrics. 1995. 96(3 Pt 2):592–594.
Article8. Jenkin GA, Chibo D, Kelly HA, Lynch PA, Catton MG. What is the cause of a rash after measles-mumps-rubella vaccination? Med J Aust. 1999. 171:194–195.
Article9. Virtanen M, Peltola H, Paunio M, Heinonen OP. Day-to-day reactogenicity and the healthy vaccinee effect of measles-mumps-rubella vaccination. Pediatrics. 2000. 106:E62.10. Chen RT, Orenstein WA. Epidemiologic methods in immunization programs. Epidemiol Rev. 1996. 18:99–117.
Article11. Forsyth K, Nagai M, Lepetic A, Trindade E. Pertussis immunization in the global pertussis initiative international region: recommended strategies and implementation considerations. Pediatr Infect Dis J. 2005. 24:5 Suppl. S93–S97.12. Galazka A. Control of pertussis in the world. World Health Stat Q. 1992. 45:238–247.13. Cook KM, Evans G. The National Vaccine Injury Compensation Program. Pediatrics. 2011. 127:Suppl 1. S74–S77.
Article14. Looker C, Kelly H. No-fault compensation following adverse events attributed to vaccination: a review of international programmes. Bull World Health Organ. 2011. 89:371–378.
Article15. Kim BY, Kim DH, Lee HJ, et al. Investigation on the frequency and severity of common adverse reactions of Japanese encephalitis vaccines. Korean J Pediatr Infect Dis. 2009. 16:183–190.
Article16. Choe YJ, Cho H, Kim SN, Bae GR, Lee JK. Serious adverse events following receipt of trivalent inactivated influenza vaccine in Korea, 2003-2010. Vaccine. 2011. 29:7727–7732.
Article17. Choe YJ, Cho H, Bae GR, Lee JK. Guillain-Barre syndrome following receipt of influenza A (H1N1) 2009 monovalent vaccine in Korea with an emphasis on Brighton Collaboration case definition. Vaccine. 2011. 29:2066–2070.
Article18. Collet JP, MacDonald N, Cashman N, Pless R. Advisory Committee on Causality Assessment. Monitoring signals for vaccine safety: the assessment of individual adverse event reports by an expert advisory committee. Bull World Health Organ. 2000. 78:178–185.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- JYNNEOS vaccine safety monitoring in the Republic of Korea, 2022: a cross-sectional study
- Effects of Nurses' Patient Safety Management Importance, Patient Safety Culture and Nursing Service Quality on Patient Safety Management Activities in Tertiary Hospitals
- Safety of influenza vaccination in children with allergic diseases
- Towards Vaccine 3.0: new era opened in vaccine research and industry
- Adenovirus Vectors: Excellent Tools for Vaccine Development