Asia Pac Allergy.
2017 Oct;7(4):221-226. 10.5415/apallergy.2017.7.4.221.
Basophils are increased and express increased levels of interleukin-4 in the parotid lesions of Kimura disease
- Affiliations
-
- 1Department of Otolaryngology, Tokyo Women's Medical University, Tokyo 162-8666, Japan. nonaka-m@twmu.ac.jp
- 2First Department of Pathology, Tokyo Women's Medical University, Tokyo 162-8666, Japan.
- 3Department of Pediatrics, Nippon Medical School, Tokyo 113-0022, Japan.
- 4Department of Otorhinolaryngology, Toto Bunkyo Hospital, Tokyo 113-0034, Japan.
- KMID: 2410216
- DOI: http://doi.org/10.5415/apallergy.2017.7.4.221
Abstract
- BACKGROUND
Kimura disease (KD) is a systemic soft-tissue disease that leads to formation of painless masses in lymph nodes, with the highest predilection for the head and neck and especially the parotid gland. KD lesions are characterized by marked eosinophil infiltration, production of IgE and increased expression of T-helper type 2 (Th2) cytokines (interleukin [IL]-4, IL-5, etc.). Skewing to a Th2 inflammation is also demonstrated in the peripheral blood, with elevated eosinophils and high IgE levels. It is thought that basophils may play important roles in orchestrating this Th2 inflammation via IL-4 production leading to the induction of IgE synthesis as well as eosinophil infiltration. However, there are no reports as yet on the role of basophils in KD.
OBJECTIVE
The present study was performed to investigate the potential role of basophils in the pathogenesis of KD. In this context we also examined the expression of IL-4 in basophils in the KD lesions.
METHODS
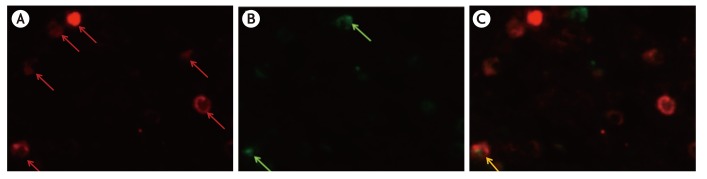
By immunohistochemistry using a monoclonal antibody against a basophil marker ProMBP1 we investigated the number and distribution of basophils in the KD lesions. By double immunohistochemistry we analyzed the colocalization of IL-4 in basophils.
RESULTS
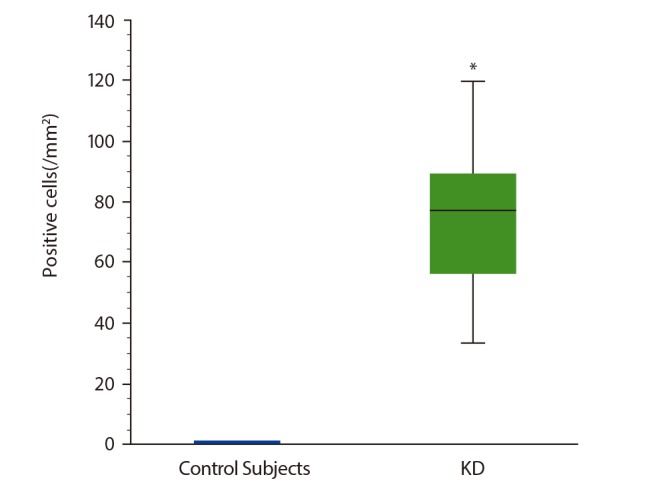
There was an increased number of basophils infiltrating the KD parotid gland lesions as compared to that in normal control parotid tissue. By double-immunofluorescence we found that approximately 7% of IL-4-positive cells in KD patients' parotid glands were basophils.
CONCLUSION
Basophils may also play a role in the pathogenesis of KD, leading to the induction of IgE synthesis and eosinophil infiltration.
MeSH Terms
Figure
Cited by 1 articles
-
Autumn leaves: about aging and allergy
Yoon-Seok Chang
Asia Pac Allergy. 2017;7(4):183-184. doi: 10.5415/apallergy.2017.7.4.183.
Reference
-
1. Kimura T, Yoshimura S, Ishikawa E. On the unusual granulation combined with hyperplastic changes of lymphatic tissues. Trans Soc Pathol Jpn. 1948; 37:179–180.2. Kimura Y, Pawankar R, Aoki M, Niimi Y, Kawana S. Mast cells and T cells in Kimura's disease express increased levels of interleukin-4, interleukin-5, eotaxin and RANTES. Clin Exp Allergy. 2002; 32:1787–1793. PMID: 12653173.
Article3. Ohta N, Fukase S, Suzuki Y, Ito T, Yoshitake H, Aoyagi M. Increase of Th2 and Tc1 cells in patients with Kimura's disease. Auris Nasus Larynx. 2011; 38:77–82. PMID: 20554415.
Article4. Bieneman AP, Chichester KL, Chen YH, Schroeder JT. Toll-like receptor 2 ligands activate human basophils for both IgE-dependent and IgE-independent secretion. J Allergy Clin Immunol. 2005; 115:295–301. PMID: 15696084.
Article5. Yanagihara Y, Kajiwara K, Basaki Y, Ikizawa K, Ebisawa M, Ra C, Tachimoto H, Saito H. Cultured basophils but not cultured mast cells induce human IgE synthesis in B cells after immunologic stimulation. Clin Exp Immunol. 1998; 111:136–143. PMID: 9472673.
Article6. Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009; 10:697–705. PMID: 19465906.
Article7. Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009; 10:706–712. PMID: 19465908.
Article8. Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009; 10:713–720. PMID: 19465907.
Article9. Sakitani E, Nonaka M, Shibata N, Furukawa T, Yoshihara T. Increased expression of thymic stromal lymphopoietin and its receptor in Kimura's disease. ORL J Otorhinolaryngol Relat Spec. 2015; 77:44–54. PMID: 25676453.
Article10. Bodger MP, Mounsey GL, Nelson J, Fitzgerald PH. A monoclonal antibody reacting with human basophils. Blood. 1987; 69:1414–1418. PMID: 2436686.
Article11. Kepley CL, Craig SS, Schwartz LB. Identification and partial characterization of a unique marker for human basophils. J Immunol. 1995; 154:6548–6555. PMID: 7759888.12. Bühring HJ, Simmons PJ, Pudney M, Müller R, Jarrossay D, van Agthoven A, Willheim M, Brugger W, Valent P, Kanz L. The monoclonal antibody 97A6 defines a novel surface antigen expressed on human basophils and their multipotent and unipotent progenitors. Blood. 1999; 94:2343–2356. PMID: 10498606.13. McEuen AR, Buckley MG, Compton SJ, Walls AF. Development and characterization of a monoclonal antibody specific for human basophils and the identification of a unique secretory product of basophil activation. Lab Invest. 1999; 79:27–38. PMID: 9952108.14. McEuen AR, Calafat J, Compton SJ, Easom NJ, Buckley MG, Knol EF, Walls AF. Mass, charge, and subcellular localization of a unique secretory product identified by the basophil-specific antibody BB1. J Allergy Clin Immunol. 2001; 107:842–848. PMID: 11344351.
Article15. Plager DA, Weiss EA, Kephart GM, Mocharla RM, Matsumoto R, Checkel JL, Schwartz LB, Gleich GJ, Leiferman KM. Identification of basophils by a mAb directed against pro-major basic protein 1. J Allergy Clin Immunol. 2006; 117:626–634. PMID: 16522463.
Article16. Macfarlane AJ, Kon OM, Smith SJ, Zeibecoglou K, Khan LN, Barata LT, McEuen AR, Buckley MG, Walls AF, Meng Q, Humbert M, Barnes NC, Robinson DS, Ying S, Kay AB. Basophils, eosinophils, and mast cells in atopic and nonatopic asthma and in late-phase allergic reactions in the lung and skin. J Allergy Clin Immunol. 2000; 105(1 Pt 1):99–107. PMID: 10629459.
Article17. Aguzzi A, Krautler NJ. Characterizing follicular dendritic cells: a progress report. Eur J Immunol. 2010; 40:2134–2138. PMID: 20853499.
Article18. Takenaka T, Okuda M, Usami A, Kawabori S, Ogami Y. Histological and immunological studies on eosinophilic granuloma of soft tissue, so-called Kimura's disease. Clin Allergy. 1976; 6:27–39. PMID: 765004.
Article19. Byers DE. Defining the roles of IL-33, thymic stromal lymphopoietin, and IL-25 in human asthma. Am J Respir Crit Care Med. 2014; 190:715–716. PMID: 25271740.
Article20. Zhou M, Ouyang W. The function role of GATA-3 in Th1 and Th2 differentiation. Immunol Res. 2003; 28:25–37. PMID: 12947222.
Article21. Bradding P, Feather IH, Wilson S, Bardin PG, Heusser CH, Holgate ST, Howarth PH. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993; 151:3853–3865. PMID: 8376806.22. Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994; 10:471–480. PMID: 8179909.
Article23. Nonaka M, Nonaka R, Woolley K, Adelroth E, Miura K, Okhawara Y, Glibetic M, Nakano K, O'Byrne P, Dolovich J, et al. Distinct immunohistochemical localization of IL-4 in human inflamed airway tissues. IL-4 is localized to eosinophils in vivo and is released by peripheral blood eosinophils. J Immunol. 1995; 155:3234–3244. PMID: 7673736.