Pediatr Gastroenterol Hepatol Nutr.
2018 Apr;21(2):101-110. 10.5223/pghn.2018.21.2.101.
Laxative Choice and Treatment Outcomes in Childhood Constipation: Clinical Data in a Longitudinal Retrospective Study
- Affiliations
-
- 1Department of Pediatrics, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand. catchari@medicine.psu.ac.th
- KMID: 2409361
- DOI: http://doi.org/10.5223/pghn.2018.21.2.101
Abstract
- PURPOSE
Functional constipation (FC) is a common gastrointestinal (GI) problem affecting children's well-being and quality of life. Although polyethylene glycol (PEG) is recommended as the first line therapy, it is not always applicable in lower socioeconomic populations. Hence, this study aimed to compare clinical courses of FC in children treated with different medications in order to identify prognostic factors related to treatment outcomes.
METHODS
We reviewed the medical records of patients aged ≤15 years diagnosed with FC according to the Rome IV criteria from 2007 to 2015 at the GI clinic, Songklanagarind Hospital. Baseline characteristic, medical history, and treatment outcomes were collected at first and subsequent visits.
RESULTS
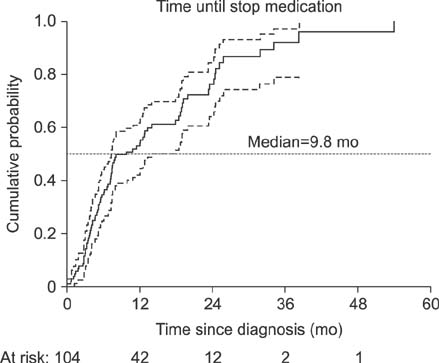
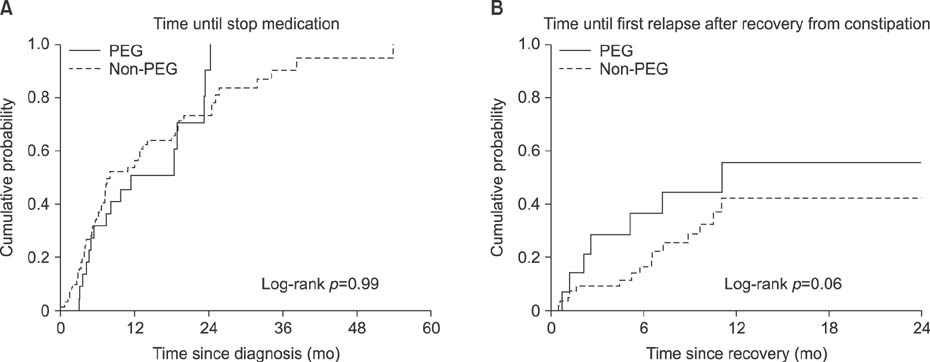
Exactly 104 patients (median age at diagnosis, 2.8 years) were diagnosed with FC. The number of follow-up visits per patient ranged from 1 to 35. The median duration of follow-up was 18.0 months (range, 6.0-84.2 months). PEG was given to 21% of patients. During the follow up period, 76% of patients experienced first recovery with a median time to recovery of 9.8 months. There were no significant differences in time until first recovery and relapse between patients who received and those who did not receive PEG (p=0.99 and 0.06, respectively). Age >6 years, normal defecation frequency, no history of cow's milk protein allergy, and use of laxatives were associated with successful outcomes.
CONCLUSION
Treatment outcomes between patients who had and never had PEG demonstrated no significant difference in our study. Hence, current practices in laxative prescriptive patterns may be effective.
MeSH Terms
Figure
Cited by 1 articles
-
Clinical Features of Severely Constipated Children: Comparison of Infrequent Bowel Movement and Fecal Soiling Groups
Gyung Lee, Jae Sung Son, Sun Hwan Bae
Pediatr Gastroenterol Hepatol Nutr. 2020;23(1):26-34. doi: 10.5223/pghn.2020.23.1.26.
Reference
-
1. van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 2006; 101:2401–2409.
Article2. Chang SH, Park KY, Kang SK, Kang KS, Na SY, Yang HR, et al. Prevalence, clinical characteristics, and man agement of functional constipation at pediatric gastroenterology clinics. J Korean Med Sci. 2013; 28:1356–1361.
Article3. Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009; 136:741–754.
Article4. Belsey J, Greenfield S, Candy D, Geraint M. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010; 31:938–949.
Article5. Youssef NN, Langseder AL, Verga BJ, Mones RL, Rosh JR. Chronic childhood constipation is associated with impaired quality of life: a case-controlled study. J Pediatr Gastroenterol Nutr. 2005; 41:56–60.
Article6. Koppen IJN, Broekaert IJ, Wilschanski M, Papadopoulou A, Ribes-Koninckx C, Thapar N, et al. Role of polyethylene glycol in the treatment of functional constipation in children. J Pediatr Gastroenterol Nutr. 2017; 65:361–363.
Article7. Afzal NA, Tighe MP, Thomson MA. Constipation in children. Ital J Pediatr. 2011; 37:28.
Article8. Gordon M, MacDonald JK, Parker CE, Akobeng AK, Thomas AG. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev. 2016; (8):CD009118.
Article9. Bongers ME, van Wijk MP, Reitsma JB, Benninga MA. Long-term prognosis for childhood constipation: clinical outcomes in adulthood. Pediatrics. 2010; 126:e156–e162.
Article10. Michaud L, Lamblin MD, Mairesse S, Turck D, Gottrand F. Outcome of functional constipation in childhood: a 10-year follow-up study. Clin Pediatr (Phila). 2009; 48:26–31.
Article11. van den Berg MM, van Rossum CH, de Lorijn F, Reitsma JB, Di Lorenzo C, Benninga MA. Functional constipation in infants: a follow-up study. J Pediatr. 2005; 147:700–704.
Article12. van Ginkel R, Reitsma JB, Büller HA, van Wijk MP, Taminiau JA, Benninga MA. Childhood constipation: longitudinal follow-up beyond puberty. Gastroenterology. 2003; 125:357–363.
Article13. Park M, Bang YG, Cho KY. Risk factors for functional constipation in young children attending daycare centers. J Korean Med Sci. 2016; 31:1262–1265.
Article14. Tam YH, Li AM, So HK, Shit KY, Pang KK, Wong YS, et al. Socioenvironmental factors associated with constipation in Hong Kong children and Rome III criteria. J Pediatr Gastroenterol Nutr. 2012; 55:56–61.
Article15. Pijpers MA, Bongers ME, Benninga MA, Berger MY. Functional constipation in children: a systematic review on prognosis and predictive factors. J Pediatr Gastroenterol Nutr. 2010; 50:256–268.
Article16. Zeevenhooven J, Koppen IJ, Benninga MA. The new Rome IV criteria for functional gastrointestinal disorders in infants and toddlers. Pediatr Gastroenterol Hepatol Nutr. 2017; 20:1–13.
Article17. Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012; 55:221–229.
Article18. R Core Team (2017). R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing;2017. 08. 31. cited 2017 Aug 31. Available from: http://www.R-project.org.19. Miele E, Simeone D, Marino A, Greco L, Auricchio R, Novek SJ, et al. Functional gastrointestinal disorders in children: an Italian prospective survey. Pediatrics. 2004; 114:73–78.
Article20. Di Lorenzo C. Pediatric anorectal disorders. Gastroenterol Clin North Am. 2001; 30:269–287, ix.
Article21. Del Ciampo IR, Galvão LC, Del Ciampo LA, Fernandes MI. Prevalence of chronic constipation in children at a primary health care unit. J Pediatr (Rio J). 2002; 78:497–502.
Article22. Loening-Baucke V, Pashankar DS. A randomized, prospective, comparison study of polyethylene glycol 3350 without electrolytes and milk of magnesia for children with constipation and fecal incontinence. Pediatrics. 2006; 118:528–535.
Article23. Modin L, Walsted AM, Rittig CS, Hansen AV, Jakobsen MS. Follow-up in childhood functional constipation: a randomized, controlled clinical trial. J Pediatr Gastroenterol Nutr. 2016; 62:594–599.24. Treepongkaruna S, Simakachorn N, Pienvichit P, Varavithya W, Tongpenyai Y, Garnier P, et al. A randomised, double-blind study of polyethylene glycol 4000 and lactulose in the treatment of constipation in children. BMC Pediatr. 2014; 14:153.
Article25. Ratanamongkol P, Lertmaharit S, Jongpiputvanich S. Polyethylene glycol 4000 without electrolytes versus milk of magnesia for the treatment of functional constipation in infants and young children: a randomized controlled trial. Asian Biomed (Res Rev News). 2009; 3:391–399.26. Dupont C, Leluyer B, Maamri N, Morali A, Joye JP, Fiorini JM, et al. Double-blind randomized evaluation of clinical and biological tolerance of polyethylene glycol 4000 versus lactulose in constipated children. J Pediatr Gastroenterol Nutr. 2005; 41:625–633.
Article27. Iacono G, Carroccio A, Cavataio F, Montalto G, Cantarero MD, Notarbartolo A. Chronic constipation as a symptom of cow milk allergy. J Pediatr. 1995; 126:34–39.
Article28. Daher S, Tahan S, Solé D, Naspitz CK, Da Silva Patrício FR, Neto UF, et al. Cow's milk protein intolerance and chronic constipation in children. Pediatr Allergy Immunol. 2001; 12:339–342.
Article29. El-Hodhod MA, Younis NT, Zaitoun YA, Daoud SD. Cow's milk allergy related pediatric constipation: appropriate time of milk tolerance. Pediatr Allergy Immunol. 2010; 21:e407–e412.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of a Constipation Intervention Program on Inpatients' Defecation
- Pharmacological treatment of chronic constipation: focused on oral laxatives
- Chronic Functional Constipation
- Polyethylene Glycol Plus Electrolytes with Stimulant Laxative in Paediatric Faecal Disimpaction: A Randomised Controlled Study
- Assessing indicators and clinical differences between functional and organic childhood constipation: a retrospective study in pediatric gastroenterology clinics