Allergy Asthma Immunol Res.
2018 May;10(3):268-277. 10.4168/aair.2018.10.3.268.
Immune Characterization of Bone Marrow-Derived Models of Mucosal and Connective Tissue Mast Cells
- Affiliations
-
- 1Department of Pediatrics, Mindich Child Health and Development Institute, Immunology Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA. sbenede@ucm.es
- KMID: 2409062
- DOI: http://doi.org/10.4168/aair.2018.10.3.268
Abstract
- PURPOSE
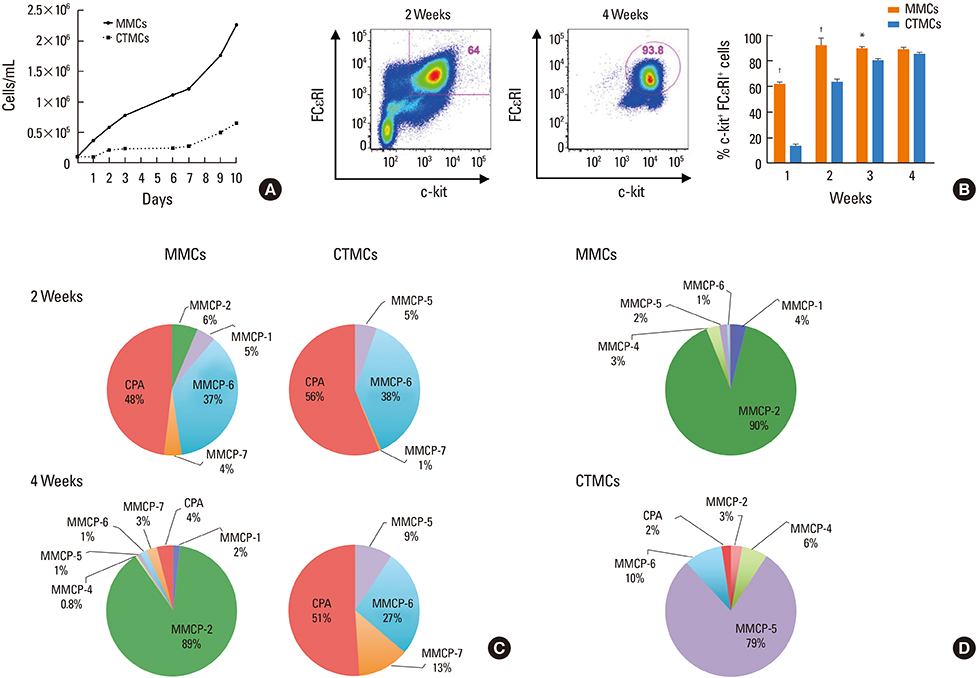
It is well appreciated that mast cells (MCs) demonstrate tissue-specific imprinting, with different biochemical and functional properties between connective tissue MCs (CTMCs) and mucosal MCs (MMCs). Although in vitro systems have been developed to model these different subsets, there has been limited investigation into the functional characteristics of the 2 major MC subsets. Here, we report the immunologic characterization of 2 MCs subsets developed in vitro from bone marrow progenitors modeling MMCs and CTMCs.
METHODS
We grew bone marrow for 4 weeks in the presence of transforming growth factor (TGF)-β, interleukin (IL)-9, IL-3, and stem cell factor (SCF) to generate MMCs, and IL-4, IL-3, and SCF to generate CTMCs.
RESULTS
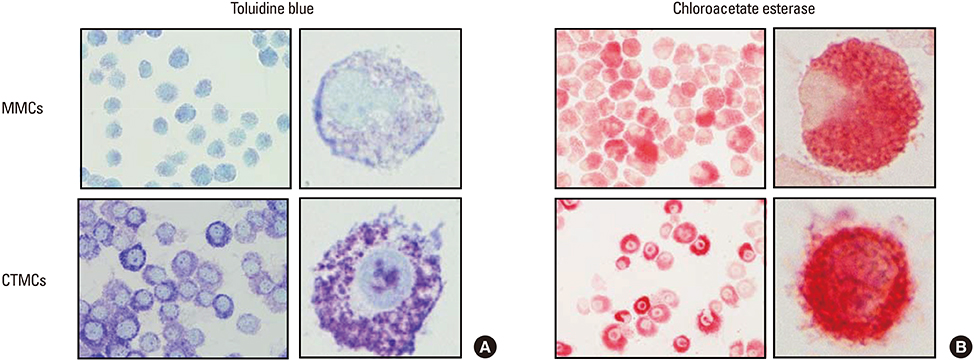
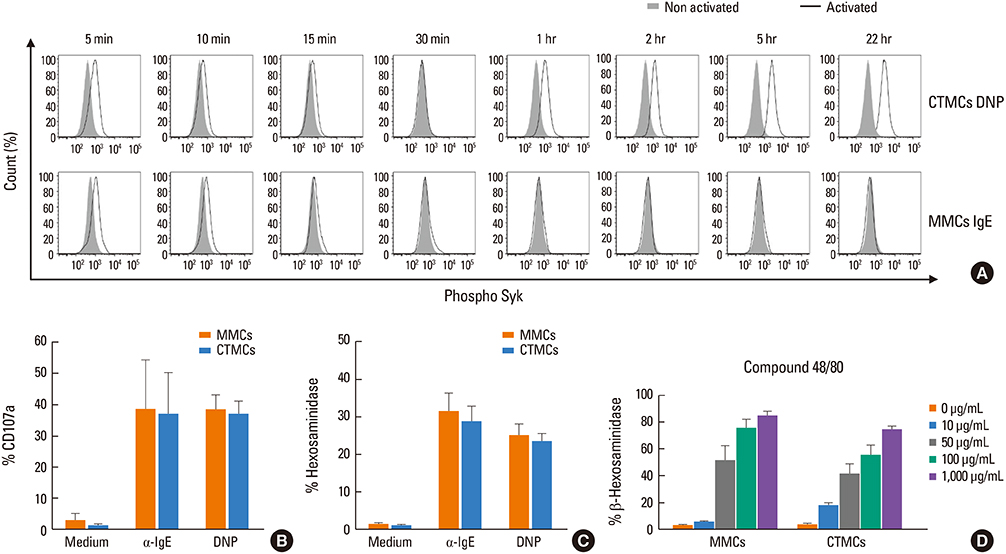
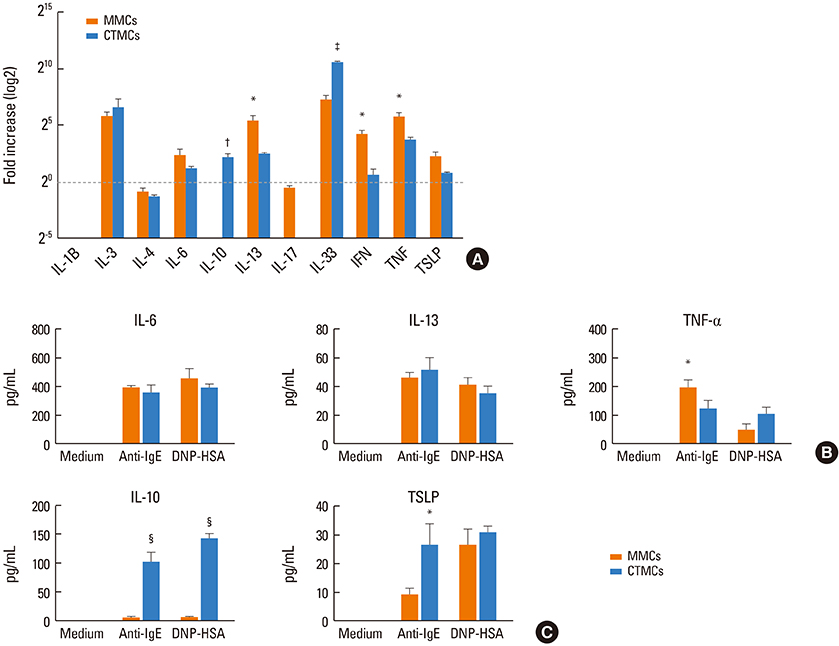
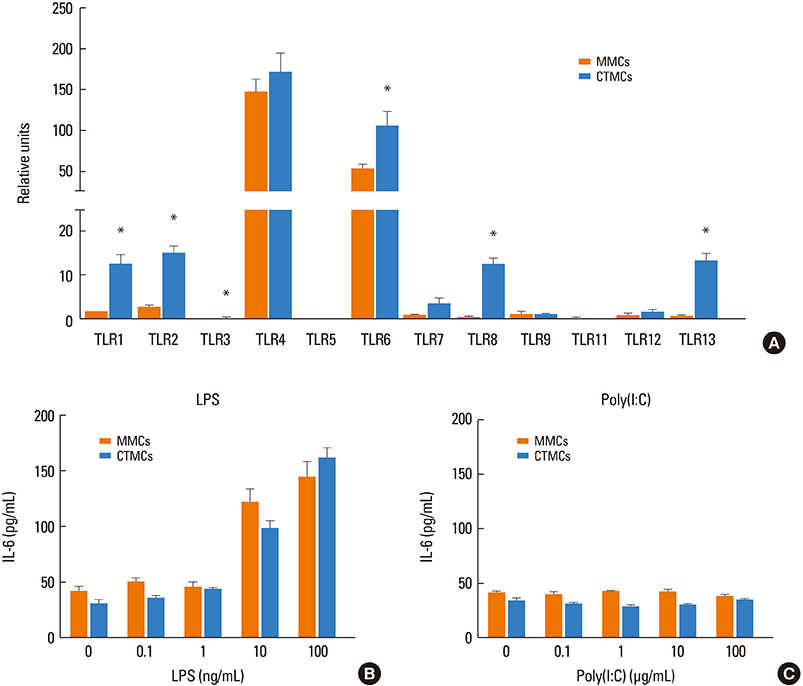
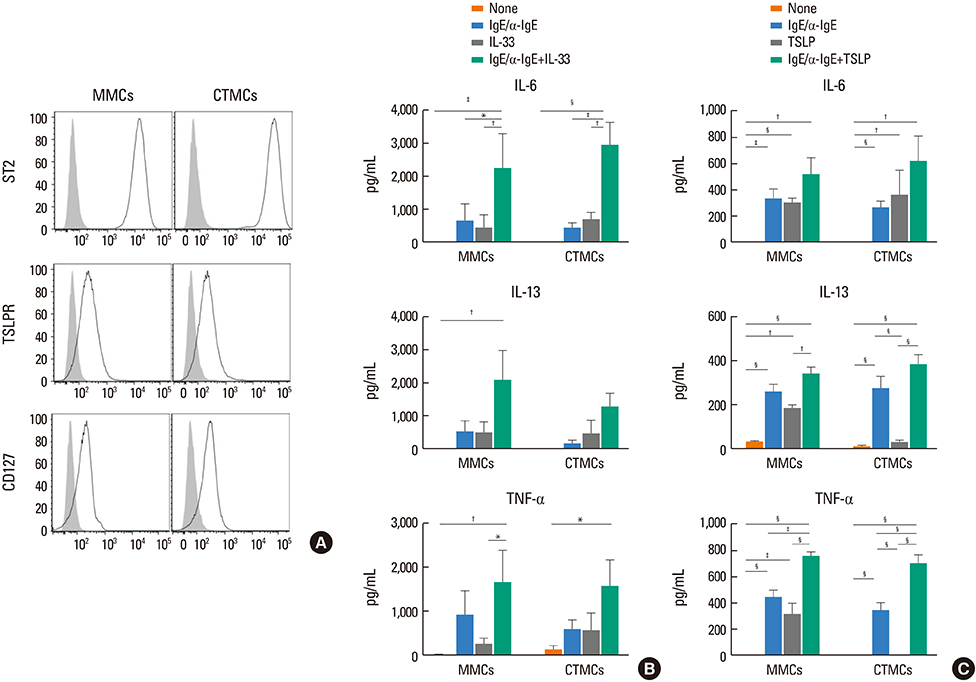
CTMCs and MMCs differed in growth rate and protease content, but their immune characteristics were remarkably similar. Both subsets responded to immunoglobulin E (IgE)-mediated activation with signaling, degranulation, and inflammatory cytokine release, although differences between subsets were noted in IL-10. CTMCs and MMCs showed a similar toll-like receptor (TLR) expression profile, dominated by expression of TLR4, TLR6, or both subsets were responsive to lipopolysaccharide (LPS), but not poly(I:C). CTMCs and MMCs express receptors for IL-33 and thymic stromal lymphopoietin (TSLP), and respond to these cytokines alone or with modified activation in response to IgE cross-linking.
CONCLUSIONS
The results of this paper show the immunologic characterization of bone marrow-derived MMCs and CTMCs, providing useful protocols for in vitro modeling of MC subsets.
MeSH Terms
-
Bone Marrow
Connective Tissue*
Cytokines
Immunoglobulin E
Immunoglobulins
In Vitro Techniques
Interleukin-10
Interleukin-3
Interleukin-33
Interleukin-4
Interleukins
Mast Cells*
Stem Cell Factor
Toll-Like Receptors
Transforming Growth Factors
Cytokines
Immunoglobulin E
Immunoglobulins
Interleukin-10
Interleukin-3
Interleukin-33
Interleukin-4
Interleukins
Stem Cell Factor
Toll-Like Receptors
Transforming Growth Factors
Figure
Reference
-
1. Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003; 112:1666–1677.
Article2. Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol. 2007; 179:6696–6703.
Article3. Reber LL, Marichal T, Mukai K, Kita Y, Tokuoka SM, Roers A, et al. Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. J Allergy Clin Immunol. 2013; 132:881–888.e1-11.
Article4. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012; 18:693–704.
Article5. Xing W, Austen KF, Gurish MF, Jones TG. Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc Natl Acad Sci U S A. 2011; 108:14210–14215.
Article6. Moon TC, St Laurent CD, Morris KE, Marcet C, Yoshimura T, Sekar Y, et al. Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol. 2010; 3:111–128.
Article7. Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol. 1997; 61:233–245.
Article8. Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012; 37:25–33.
Article9. Galli SJ, Tsai M, Marichal T, Tchougounova E, Reber LL, Pejler G. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. 2015; 126:45–127.10. Bryce PJ, Falahati R, Kenney LL, Leung J, Bebbington C, Tomasevic N, et al. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J Allergy Clin Immunol. 2016; 138:769–779.
Article11. Sellge G, Bischoff SC. Isolation, culture, and characterization of intestinal mast cells. Methods Mol Biol. 2006; 315:123–138.
Article12. Kulka M, Metcalfe DD. Isolation of tissue mast cells. Curr Protoc Immunol. 2010; Chapter 7:Unit 7.25.
Article13. Jensen BM, Swindle EJ, Iwaki S, Gilfillan AM. Generation, isolation, and maintenance of rodent mast cells and mast cell lines. Curr Protoc Immunol. 2006; Chapter 3:Unit 3.23.
Article14. Stassen M, Arnold M, Hültner L, Müller C, Neudörfl C, Reineke T, et al. Murine bone marrow-derived mast cells as potent producers of IL-9: costimulatory function of IL-10 and kit ligand in the presence of IL-1. J Immunol. 2000; 164:5549–5555.15. Vukman KV, Visnovitz T, Adams PN, Metz M, Maurer M, O'Neill SM. Mast cells cultured from IL-3-treated mice show impaired responses to bacterial antigen stimulation. Inflamm Res. 2012; 61:79–85.
Article16. Kovarova M. Isolation and characterization of mast cells in mouse models of allergic diseases. Methods Mol Biol. 2013; 1032:109–119.
Article17. Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996; 135:279–290.
Article18. Rådinger M, Jensen BM, Swindle E, Gilfillan AM. Assay of mast cell mediators. Methods Mol Biol. 2015; 1220:307–323.
Article19. Eklund KK, Ghildyal N, Austen KF, Stevens RL. Induction by IL-9 and suppression by IL-3 and IL-4 of the levels of chromosome 14-derived transcripts that encode late-expressed mouse mast cell proteases. J Immunol. 1993; 151:4266–4273.20. Miller HR, Wright SH, Knight PA, Thornton EM. A novel function for transforming growth factor-beta1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific beta-chymase, mouse mast cell protease-1. Blood. 1999; 93:3473–3486.21. Frandsen PM, Krohn IJ, Hoffmann HJ, Schiøtz PO. The influence of IgE on cultured human mast cells. Allergy Asthma Immunol Res. 2013; 5:409–414.
Article22. Friend DS, Ghildyal N, Gurish MF, Hunt J, Hu X, Austen KF, et al. Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J Immunol. 1998; 160:5537–5545.23. Stevens RL, Friend DS, McNeil HP, Schiller V, Ghildyal N, Austen KF. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc Natl Acad Sci U S A. 1994; 91:128–132.
Article24. Hunt JE, Stevens RL, Austen KF, Zhang J, Xia Z, Ghildyal N. Natural disruption of the mouse mast cell protease 7 gene in the C57BL/6 mouse. J Biol Chem. 1996; 271:2851–2855.
Article25. Heutinck KM, ten Berge IJ, Hack CE, Hamann J, Rowshani AT. Serine proteases of the human immune system in health and disease. Mol Immunol. 2010; 47:1943–1955.
Article26. Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010; 115:4981–4990.
Article27. Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007; 95:167–255.
Article28. Irani AM, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB. Human mast cell carboxypeptidase. Selective localization to MCTC cells. J Immunol. 1991; 147:247–253.29. Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986; 83:4464–4468.
Article30. Feng LL, Gao JM, Li PP, Wang X. IL-9 contributes to immunosuppression mediated by regulatory T cells and mast cells in B-cell non-hodgkin's lymphoma. J Clin Immunol. 2011; 31:1084–1094.
Article31. Broome M, Villarreal B. Differential staining of mast cells with toluidine blue. J Histotechnol. 2012; 35:27–30.
Article32. Ikawati Z, Hayashi M, Nose M, Maeyama K. The lack of compound 48/80-induced contraction in isolated trachea of mast cell-deficient Ws/Ws rats in vitro: the role of connective tissue mast cells. Eur J Pharmacol. 2000; 402:297–306.33. Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012; 12:168–179.
Article34. Sandig H, Bulfone-Paus S. TLR signaling in mast cells: common and unique features. Front Immunol. 2012; 3:185.
Article35. McCurdy JD, Olynych TJ, Maher LH, Marshall JS. Cutting edge: distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003; 170:1625–1629.
Article36. Okumura S, Kashiwakura J, Tomita H, Matsumoto K, Nakajima T, Saito H, et al. Identification of specific gene expression profiles in human mast cells mediated by Toll-like receptor 4 and Fcepsilon-RI. Blood. 2003; 102:2547–2554.37. Varadaradjalou S, Féger F, Thieblemont N, Hamouda NB, Pleau JM, Dy M, et al. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol. 2003; 33:899–906.38. Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004; 114:174–182.
Article39. Kulka M, Metcalfe DD. TLR3 activation inhibits human mast cell attachment to fibronectin and vitronectin. Mol Immunol. 2006; 43:1579–1586.
Article40. Yoshioka M, Fukuishi N, Iriguchi S, Ohsaki K, Yamanobe H, Inukai A, et al. Lipoteichoic acid downregulates FcepsilonRI expression on human mast cells through Toll-like receptor 2. J Allergy Clin Immunol. 2007; 120:452–461.41. McCurdy JD, Lin TJ, Marshall JS. Toll-like receptor 4-mediated activation of murine mast cells. J Leukoc Biol. 2001; 70:977–984.42. Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J Immunol. 2001; 167:2250–2256.
Article43. Masuda A, Yoshikai Y, Aiba K, Matsuguchi T. Th2 cytokine production from mast cells is directly induced by lipopolysaccharide and distinctly regulated by c-Jun N-terminal kinase and p38 pathways. J Immunol. 2002; 169:3801–3810.
Article44. Ikeda T, Funaba M. Altered function of murine mast cells in response to lipopolysaccharide and peptidoglycan. Immunol Lett. 2003; 88:21–26.
Article45. Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol. 2004; 173:531–541.
Article46. Li G, Domenico J, Jia Y, Lucas JJ, Gelfand EW. NF-kappaB-dependent induction of cathelicidin-related antimicrobial peptide in murine mast cells by lipopolysaccharide. Int Arch Allergy Immunol. 2009; 150:122–132.
Article47. Mrabet-Dahbi S, Metz M, Dudeck A, Zuberbier T, Maurer M. Murine mast cells secrete a unique profile of cytokines and prostaglandins in response to distinct TLR2 ligands. Exp Dermatol. 2009; 18:437–444.
Article48. Saluja R, Delin I, Nilsson GP, Adner M. FcεR1-mediated mast cell reactivity is amplified through prolonged Toll-like receptor-ligand treatment. PLoS One. 2012; 7:e43547.
Article49. Blázquez AB, Berin MC. Microbiome and food allergy. Transl Res. 2017; 179:199–203.
Article50. Blázquez AB, Mayer L, Berin MC. Thymic stromal lymphopoietin is required for gastrointestinal allergy but not oral tolerance. Gastroenterology. 2010; 139:1301–1309.
Article51. Tordesillas L, Goswami R, Benedé S, Grishina G, Dunkin D, Järvinen KM, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest. 2014; 124:4965–4975.
Article52. Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013; 131:187–200.
Article53. Han H, Thelen TD, Comeau MR, Ziegler SF. Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J Clin Invest. 2014; 124:5442–5452.
Article54. Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie AN, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol. 2016; 138:1356–1366.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bladder Mucosal Mast Cell Response in Bladder Tumor
- Role of Mast Cells in Allergic Inflammation and Innate Immunity
- The Role of Mast Cell in Hyperlaxity of Conjunctiva
- Generation and Characterization of Alloenic Radiation Bone Marrow Chimera
- Distribution of Mast Cells in the Rat Tympanic Membrane and Eustachian Tube