Investig Magn Reson Imaging.
2018 Mar;22(1):26-36. 10.13104/imri.2018.22.1.26.
Quantitative Evaluation of the First Order Creatine-Kinase Reaction Rate Constant in in vivo Shunted Ovine Heart Treated with Oxandrolone Using Magnetization Transfer 31P Magnetic Resonance Spectroscopy (MT-31P-MRS) and 1H/31P Double-Tuned Surface Coil: a Preliminary Study
- Affiliations
-
- 1Utah Center for Advanced Imaging Research, University of Utah, Utah, USA. ekj@ucair.med.utah.edu
- 2Department of Physics and Astronomy, University of Utah, Utah, USA.
- 3Department of Pediatrics, University of Utah, Utah, USA.
- 4Department of Radiology and Imaging Sciences, University of Utah, Utah, USA.
- 5Department of Surgery, University of Utah, Utah, USA.
- KMID: 2408815
- DOI: http://doi.org/10.13104/imri.2018.22.1.26
Abstract
- PURPOSE
Children born with single ventricle physiology demonstrate poor growth rate and suffer from malnutrition, which lead to increased morbidity and mortality in this population. We assume that an anabolic steroid, oxandrolone, will promote growth in these infants by improving myocardial energy utilization. The purpose of this paper is to study the efficacy of oxandrolone on myocardial energy consumption in these infants.
MATERIALS AND METHODS
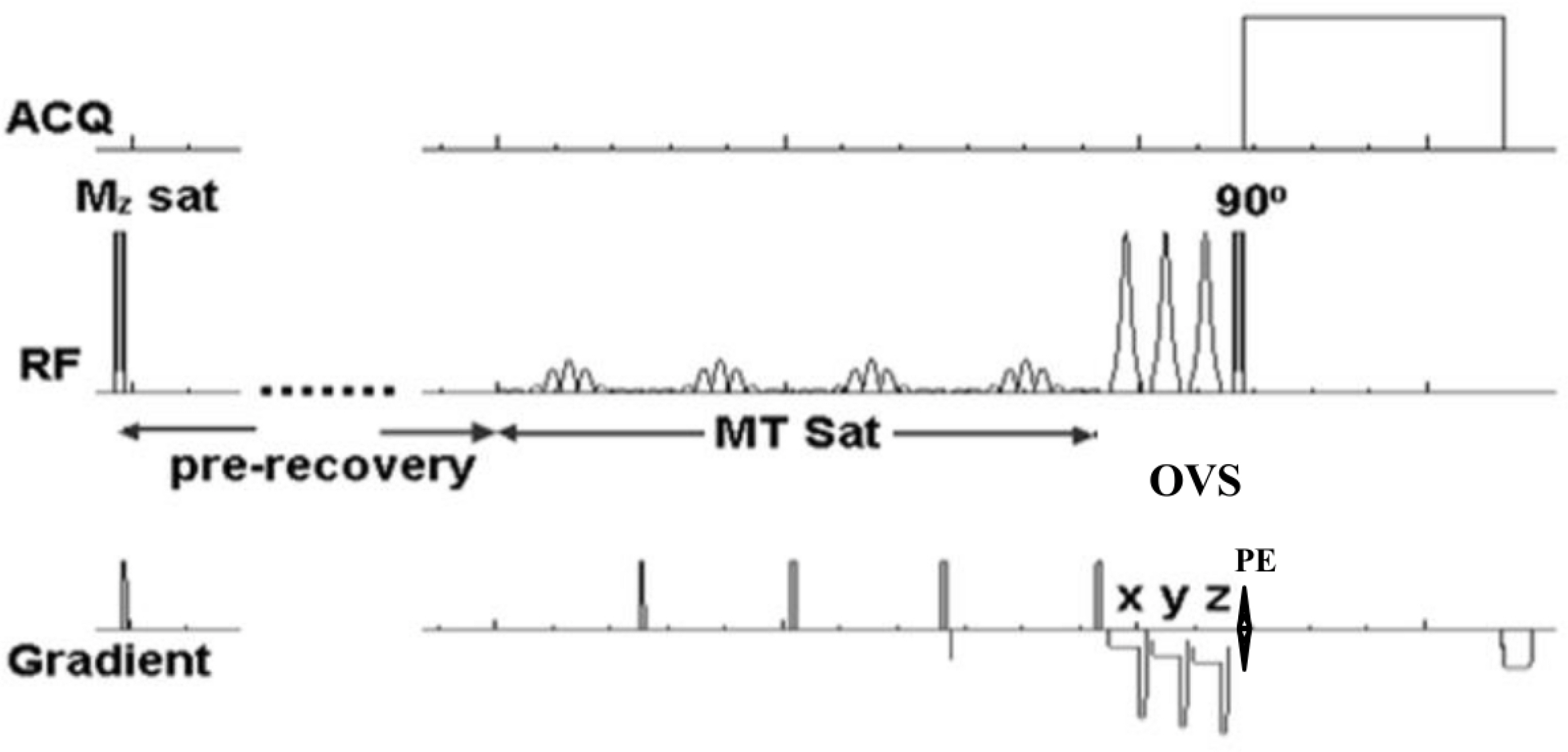
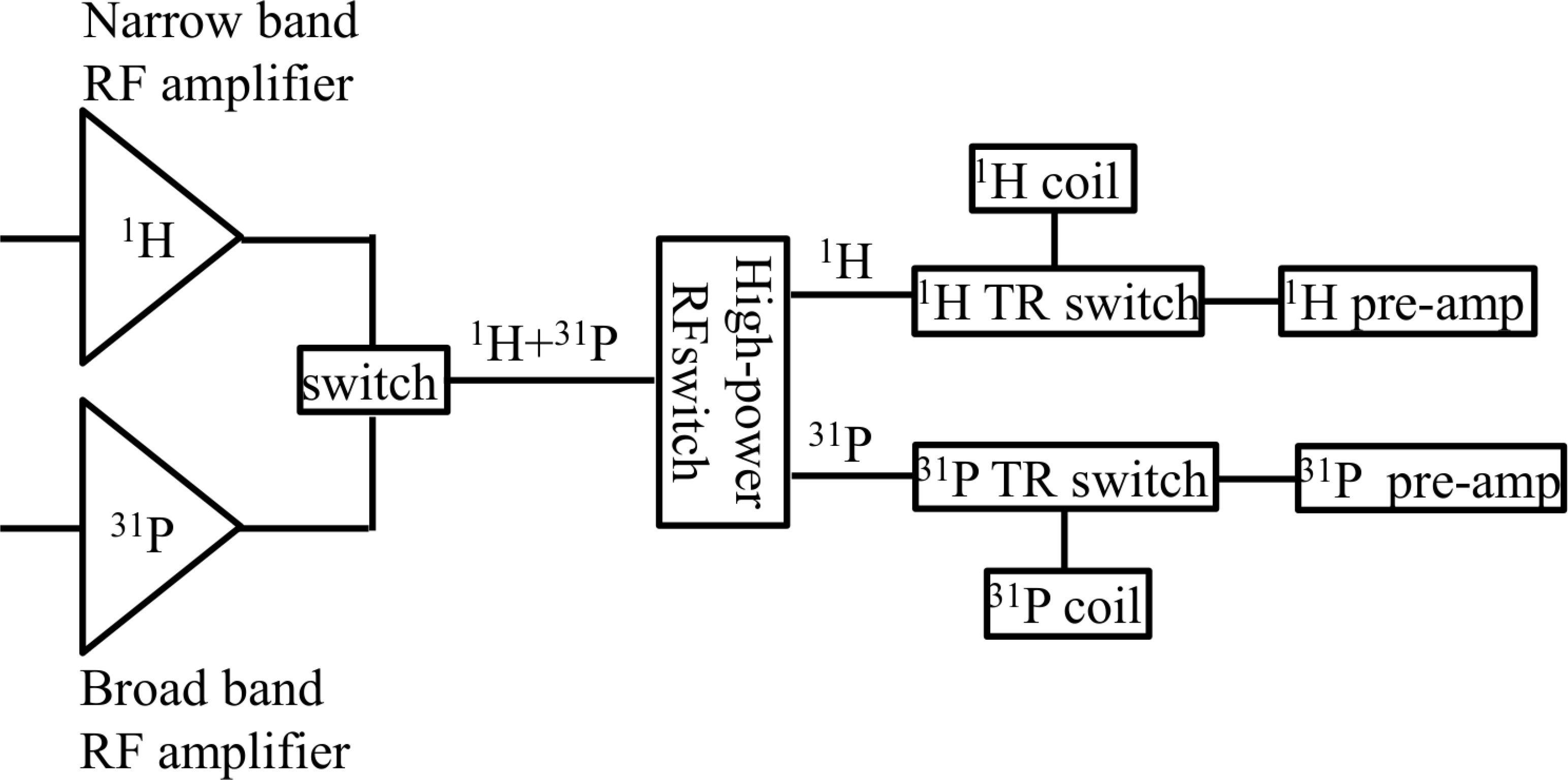
We modeled single ventricle physiology in a lamb by prenatally shunting the aorta to the pulmonary artery and then postnatally, we monitored cardiac energy utilization by quantitatively measuring the first order reaction rate constant, kf of the creatine-kinase reaction in the heart using magnetization transfer 31P magnetic resonance spectroscopy, home built 1H/31P transmit/receive double tuned coil, and transmit/receive switch. We also performed cine MRI to study the structure and dynamic function of the myocardium and the left ventricular chamber. The spectroscopy data were processed using home-developed python software, while cine data were analyzed using Argus software.
RESULTS
We quantitatively measured both the first order reaction rate constant and ejection fraction in the control, shunted, and the oxandrolone-treated lambs. Both kf and ejection fraction were found to be more significantly reduced in the shunted lambs compared to the control lambs, and they are increased in oxandrolone-treated lambs.
CONCLUSION
Some improvement was observed in both the first order reaction rate constant and ejection fraction for the lamb treated with oxandrolone in our preliminary study.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Hancock Friesen CL, Forbess JM. Surgical management of the single ventricle. Prog Pediatr Cardiol. 2002; 16:47–68.
Article2. Schwalbe-Terilli CR, Hartman DH, Nagle ML, et al. Enteral feeding and caloric intake in neonates after cardiac surgery. Am J Crit Care. 2009; 18:52–57.
Article3. Anderson JB, Beekman RH 3rd, Eghtesady P, et al. Predictors of poor weight gain in infants with a single ventricle. J Pediatr. 2010; 157:407–413. 413 e401.
Article4. Nydegger A, Bines JE. Energy metabolism in infants with congenital heart disease. Nutrition. 2006; 22:697–704.
Article5. Leitch CA. Growth, nutrition and energy expenditure in pediatric heart failure. Prog Pediatr Cardiol. 2000; 11:195–202.
Article6. Di Maria MV, Glatz AC, Ravishankar C, et al. Supplemental tube feeding does not mitigate weight loss in infants with shunt-dependent single-ventricle physiology. Pediatr Cardiol. 2013; 34:1350–1356.
Article7. Fox-Wheeler S, Heller L, Salata CM, et al. Evaluation of the effects of oxandrolone on malnourished HIV-positive pediatric patients. Pediatrics. 1999; 104:e73.
Article8. Porro LJ, Herndon DN, Rodriguez NA, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg. 2012; 214:489–502. discussion 502–484.
Article9. Rosenfeld RG, Frane J, Attie KM, et al. Six-year results of a randomized, prospective trial of human growth hormone and oxandrolone in Turner syndrome. J Pediatr. 1992; 121:49–55.
Article10. Wilson DM, McCauley E, Brown DR, Dudley R. Oxandrolone therapy in constitutionally delayed growth and puberty. BioTechnology General Corporation Cooperative Study Group. Pediatrics. 1995; 96:1095–1100.11. Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001; 233:556–564.
Article12. Przkora R, Jeschke MG, Barrow RE, et al. Metabolic and hormonal changes of severely burned children receiving longterm oxandrolone treatment. Ann Surg. 2005; 242:384–389. discussion 390–381.
Article13. Wolf SE, Thomas SJ, Dasu MR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003; 237:801–810. discussion 810–801.
Article14. Reddy VM, Meyrick B, Wong J, et al. In utero placement of aortopulmonary shunts. A model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation. 1995; 92:606–613.15. Neubauer S. The failing heart–an engine out of fuel. N Engl J Med. 2007; 356:1140–1151.16. Bottomley PA, Hardy CJ. Mapping creatine kinase reaction rates in human brain and heart with 4 tesla saturation transfer 31P NMR. J Magn Reson. 1992; 99:443–448.
Article17. Bottomley PA, Ouwerkerk R, Lee RF, Weiss RG. Four-angle saturation transfer (FAST) method for measuring creatine kinase reaction rates in vivo. Magn Reson Med. 2002; 47:850–863.
Article18. Xiong Q, Li Q, Mansoor A, et al. Novel strategy for measuring creatine kinase reaction rate in the in vivo heart. Am J Physiol Heart Circ Physiol. 2009; 297:H1010–1019.
Article19. Schar M, El-Sharkawy AM, Weiss RG, Bottomley PA. Triple repetition time saturation transfer (TRiST) 31P spectroscopy for measuring human creatine kinase reaction kinetics. Magn Reson Med. 2010; 63:1493–1501.20. Schar M, Gabr RE, El-Sharkawy AM, Steinberg A, Bottomley PA, Weiss RG. Two repetition time saturation transfer (TwiST) with spill-over correction to measure creatine kinase reaction rates in human hearts. J Cardiovasc Magn Reson. 2015; 17:70.
Article21. Bashir A, Gropler R. Reproducibility of creatine kinase reaction kinetics in human heart: a (31) P time-dependent saturation transfer spectroscopy study. NMR Biomed. 2014; 27:663–671.22. Clarke WT, Robson MD, Neubauer S, Rodgers CT. Creatine kinase rate constant in the human heart measured with 3D-localization at 7 tesla. Magn Reson Med. 2017; 78:20–32.
Article23. Ugurbil K. Magnetization-transfer measurements of individual rate constants in the presence of multiple reactions. J Magn Reson. 1985; 64:207–219.24. Kingsley-Hickman PB, Sako EY, Mohanakrishnan P, et al. 31P NMR studies of ATP synthesis and hydrolysis kinetics in the intact myocardium. Biochemistry. 1987; 26:7501–7510.25. Kingsley PB, Monahan WG. Corrections for off-resonance effects and incomplete saturation in conventional (two-site) saturation-transfer kinetic measurements. Magn Reson Med. 2000; 43:810–819.
Article26. Jeong EK, Sung YH, Kim SE, et al. Measurement of creatine kinase reaction rate in human brain using magnetization transfer image-selected in vivo spectroscopy (MT-ISIS) and a volume 31P/1H radiofrequency coil in a clinical 3-T MRI system. NMR Biomed. 2011; 24:765–770.27. Potter W, Wang L, McCully K, Zhao Q. Evaluation of a new 1H/31P dual-tuned birdcage coil for 31P spectroscopy. Concepts Magn Reson Part B Magn Reson Eng. 2013; 43:90–99.28. Thapa B, Dahl M, Frank D, Burch P, Jeong EK. Quantitaive evaluation of the first order rate constant of creatine-kinase reaction in ovine heart using magntization transfer 31P magnetic resonance spectroscopy (MT-31P-MRS). In Proceedings of the 23rd Scientific Meeting of International Society for Magnetic Resonance in Medicine. Toronto. 2015. 2003.29. Thapa B, Kaggie J, Sapkota N, Frank D, Jeong EK. Design and development of a general-purpose transmit/receive (T/R) switch for MRI, compatible for a linear, quadrature and double-tuned RF coil. Concepts Magn Reson Part B Magn Reson Eng. 2016; 46B:56–65.30. Adriany G, Gruetter R. A half-volume coil for efficient proton decoupling in humans at 4 tesla. J Magn Reson. 1997; 125:178–184.
Article31. Kaggie JD, Hadley JR, Badal J, et al. A 3 T sodium and proton composite array breast coil. Magn Reson Med. 2014; 71:2231–2242.
Article32. Thapa B, Kaggie J, Sapkota N, Jeong EK. Design and develpoment of general purpose transmit-receive (TR) switch for a linear, quadrature and dual tuned coils. In Proceedings of the 23rd Scientific Meeting of International Society for Magnetic Resonance in Medicine. Toronto. 2015. 1784.33. Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005; 102:808–813.
Article34. Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation. 2006; 114:1151–1158.
Article35. Abraham MR, Bottomley PA, Dimaano VL, et al. Creatine kinase adenosine triphosphate and phosphocreatine energy supply in a single kindred of patients with hypertrophic cardiomyopathy. Am J Cardiol. 2013; 112:861–866.
Article36. Nascimben L, Friedrich J, Liao R, Pauletto P, Pessina AC, Ingwall JS. Enalapril treatment increases cardiac performance and energy reserve via the creatine kinase reaction in myocardium of Syrian myopathic hamsters with advanced heart failure. Circulation. 1995; 91:1824–1833.
Article37. Chesky JA, Rockstein M, Lopez T. Changes with age of myocardial creatine phosphokinase in the male Fischer rat. Mech Ageing Dev. 1980; 12:237–243.
Article38. Chen C, Guerrero JL, Vazquez de Prada JA, et al. Intracardiac ultrasound measurement of volumes and ejection fraction in normal, infarcted, and aneurysmal left ventricles using a 10-MHz ultrasound catheter. Circulation. 1994; 90:1481–1491.
Article39. Wisneski JA, Pfeil CN, Wyse DG, Mitchell R, Rahimtoola SH, Gertz EW. Left ventricular ejection fraction calculated from volumes and areas: underestimation by area method. Circulation. 1981; 63:149–151.
Article40. Rich S, Chomka EV, Stagl R, Shanes JG, Kondos GT, Brundage BH. Determination of left ventricular ejection fraction using ultrafast computed tomography. Am Heart J. 1986; 112:392–396.
Article41. Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000; 21:1387–1396.
Article42. Malayeri AA, Johnson WC, Macedo R, Bathon J, Lima JA, Bluemke DA. Cardiac cine MRI: quantification of the relationship between fast gradient echo and steady-state free precession for determination of myocardial mass and volumes. J Magn Reson Imaging. 2008; 28:60–66.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Nuclear Overhauser Enhancement in Liver and Heart 31P NMR Spectra Localized by 2D Chemical Shift Technique
- Human in-vivo 31P MR Spectroscopy of Benign and Malignant Breast Tumors
- Use of in vivo magnetic resonance spectroscopy for studying metabolic diseases
- Metabolic Abnormalities in Patients with Mitochondrial Myopathy Evaluated by In Vivo 31P Magnetic Resonance Spectroscopy
- Comparative of P spectroscopy and histochemical mapping in myocardial infarction in cats