Korean J Radiol.
2001 Jun;2(2):80-86. 10.3348/kjr.2001.2.2.80.
Human in-vivo 31P MR Spectroscopy of Benign and Malignant Breast Tumors
- KMID: 754109
- DOI: http://doi.org/10.3348/kjr.2001.2.2.80
Abstract
OBJECTIVE
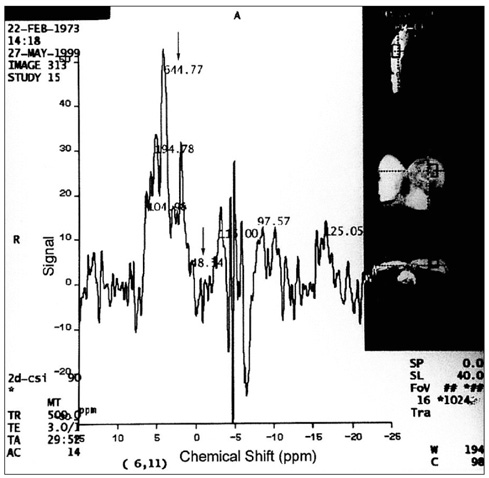
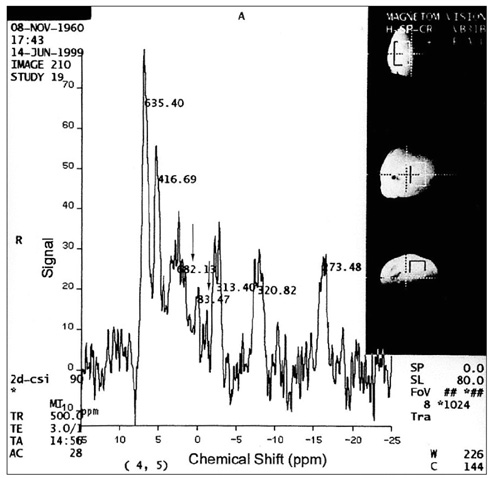
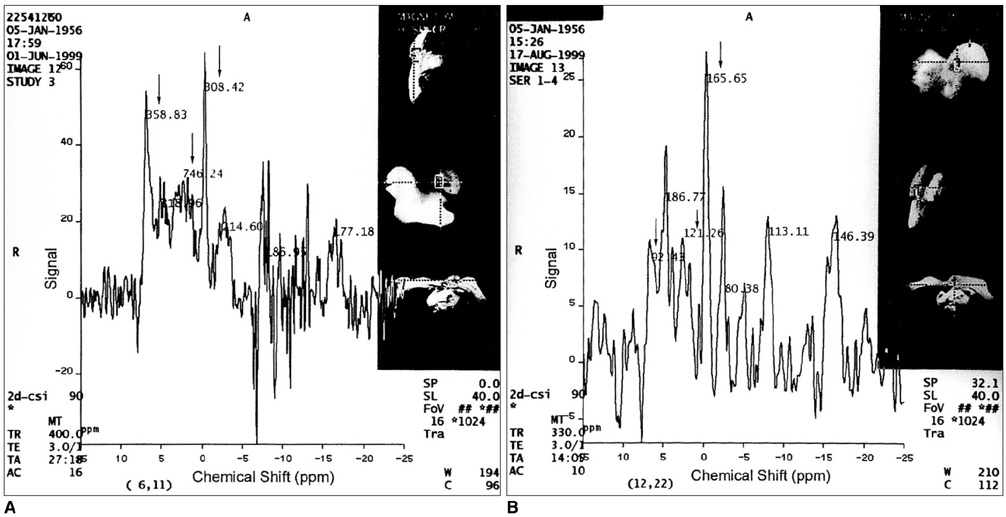
To assess the potential clinical utility of in-vivo 31P magnetic resonance spectroscopy (MRS) in patients with various malignant and benign breast lesions. MATERIALS AND METHODS: Seventeen patients with untreated primary malignant breast lesions (group I), eight patients with untreated benign breast lesions (group II) and seven normal breasts (group III) were included in this study. In-vivo 31P MRS was performed using a 1.5 Tesla MR scanner. Because of the characteristics of the coil, the volume of the tumor had to exceed 12 cc (3 x 2 x 2 cm), with a superoinferior diameter at least 3 cm. Mean and standard deviations of each metabolite were calculated and metabolite ratios, such as PME/PCr, PDE/PCr, T-ATP/PCr and PCr/T-ATP were calculated and statistically analyzed. RESULTS: Significant differences in PME were noted between groups I and III (p=0.0213), and between groups II and III (p=0.0213). The metabolite ratios which showed significant differences were PME/PCr (between groups II and III) (p=0.0201), PDE/PCr (between groups I and III, and between groups II and III) (p=0.0172), T-ATP/PCr (between groups II and III) (p=0.0287), and PCr/T-ATP (between groups II and III) (p=0.0287). There were no significant parameters between groups I and II. CONCLUSION: In-vivo 31P MRS is not helpful for establishing a differential diagnosis between benign and malignant breast lesions, at least with relatively large lesions greater than 3 cm in one or more dimensions.
Keyword
MeSH Terms
Figure
Reference
-
1. Aisen AM, Chenevert TL. MR spectroscopy : clinical perspective. Radiology. 1989. 173:593–599.2. Bottomley PA. Human in-vivo NMR spectroscopy in diagnostic medicine : Clinical tool or research probe? Radiology. 1989. 170:1–15.3. Merchant TE. MR spectroscopy of the breast. Magn Reson Imaging Clin N Am. 1994. 2:691–703.4. Yoon K-H, Lee M-G, Lee JH, Kim CG, Auh YH. In-vivo 31P magnetic resonance spectroscopy in liver cirrhosis : assessment of phosphorus metabolites according to hepatic dysfunction. J Korean Radiol Soc. 1998. 39:1171–1176.5. Oberhaensli RD, Hilton-Jones D, Bore PJ, et al. Biochemical investigation of human tumors in vivo with phosphorus-31 magnetic resonance spectroscopy. Lancet. 1986. 2(8497):8–11.6. Weinreb JC, Newstead G. MR imaging of the breast. Radiology. 1995. 196:593–610.7. Kaplan O, Cohen JS. Metabolism of breast cancer cells as revealed by non-invasive magnetic resonance spectroscopy studies. Breast Cancer Res Treat. 1994. 31:285–299.8. Kvistad KA, Bakken IJ, Gribbestad IS, et al. Characterization of neoplastic and normal human breast tissues with in-vivo 1H MR spectroscopy. J Magn Reson Imaging. 1999. 10:159–164.9. Mackinnon WB, Barry PA, Malycha PL, et al. Fine-needle biopsy specimens of benign breast lesions distinguished from invasive cancer ex vivo with proton MR spectroscopy. Radiology. 1997. 204:661–666.10. Roebuck JR, Cecil KM, Schnall MD, Lenkinski RE. Human breast lesions: Characterization with proton MR spectroscopy. Radiology. 1998. 209:269–275.11. Gribbestad IS, Singstad TE, Nilsen G, et al. In-vivo 1H MRS of normal breast and breast tumors using a dedicated double breast coil. J Magn Reson Imaging. 1998. 8:1191–1197.12. Gribbestad IS, Petersen SB, Fjosne HE, Kvinnsland S, Krane J. 1H NMR spectroscopic characterization of perchloric acid extracts from breast carcinomas and non-involved breast tissue. NMR Biomed. 1994. 7:181–194.13. Merchant TE, Thelissen GRP, Graaf PW, Otter WD, Glonek T. Clinical magnetic resonance spectroscopy of human breast disease. Invest Radiol. 1991. 26:1053–1059.14. Kalra R, Wade KE, Hands L, et al. Phosphomonoester is associated with proliferation in human breast cancer : a 31P MRS study. Br J Cancer. 1993. 67:1145–1153.15. Sijen PE, Wijrdeman HK, Moerland MA, et al. Human breast cancer in vivo:H-1 and P-31 MR spectroscopy at 1.5T. Radiology. 1988. 169:615–620.16. Twelves CJ, Porter DA, Lowry M, et al. Phosphorus-31 metabolism of post-menopausal breast cancer studied in vivo by magnetic resonance spectroscopy. Br J Cancer. 1994. 69:1151–1156.17. Leach MO, Verrill M, Glaholm J, et al. Measurements of human breast cancer using magnetic resonance spectroscopy : a review of clinical measurements and a report of localized 31P measurements of response to treatment. NMR Biomed. 1998. 11:314–340.18. Redmond OM, Stack JP, O'Conner NG, et al. In-vivo Phosphorus-31 magnetic resonance spectroscopy of normal and pathological breast tissues. Br J Radiol. 1991. 64:210–216.19. Smith TAD, Bush C, Jameson C, et al. Phospholipid metabolites, prognosis and proliferation in human breast carcinoma. NMR Biomed. 1993. 6:318–323.20. Degani H, Furman E, Fields S. Magnetic resonance imaging and spectroscopy of MCF7 human breast cancer:pathophysiology and monitoring of treatment. Clin Chim Acta. 1994. 228:19–33.21. Singer S, Souza K, Thilly WG. Pyruvate utilization, phosphocholine and adenosine triphosphate(ATP) are markers of human breast tumor progression: a 31P- and 13C-nuclear magnetic resonance( NMR) spectroscopy study. Cancer Res. 1995. 55(22):5140–5145.22. Merchant TE, Meneses P, Gierke LW, Otter WD, Glonek T. 31P Magnetic resonance phospholipid profiles of neoplastic human breast tissues. Br J Cancer. 1991. 63:693–698.23. Twelves CJ, Lowry M, Porter DA, et al. Phosphorus-31 metabolism of human breast - an in-vivo magnetic resonance spectroscopic study at 1.5T. Br J Radiol. 1994. 67:36–45.24. Degani H, Horowitz A, Itzchak Y. Breast tumors:evaluation with P-31 MR spectroscopy. Radiology. 1986. 161:53–55.25. Merchant TE, Gierke LW, Meneses P, Glonek T. 31P magnetic resonance spectroscopic profiles of neoplastic human breast tissues. Cancer Res. 1988. 48:5112–5118.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vivo31P MR Spectroscopy of Breast Tumors: Preliminary Results
- Localized, water-suppressed in vivo H MR spectroscopy of human brain tumors: Preliminary results
- In vivo H MR spectroscopy of human brain in six normal volunteers
- In-vivo Proton Magnetic Resonance Spectroscopy in Adnexal Lesions
- In Vivo 31P Magnetic Resonance Spectroscopy in Liver Cirrhosis: Assessment of Phosphorus Metabolites accordingto Hepatic Dysfunction