Korean J Radiol.
2002 Jun;3(2):105-112. 10.3348/kjr.2002.3.2.105.
In-vivo Proton Magnetic Resonance Spectroscopy in Adnexal Lesions
- Affiliations
-
- 1Department of Radiology, Inha University College of Medicine, Korea. suhchae@inha.ac.kr
- 2Department of Radiology, Hallym University College of Medicine, Hangang Sacred Heart Hospital, Korea.
- 3NMR Laboratory, Asan Institute for Life Sciences, Korea.
- 4Department of Obstetrics and Gynecology, Inha University College of Medicine, Korea.
- KMID: 754069
- DOI: http://doi.org/10.3348/kjr.2002.3.2.105
Abstract
OBJECTIVE
To explore the in-vivo 1H- MR spectral features of adnexal lesions and to characterize the spectral patterns of various pathologic entities.
MATERIALS AND METHODS
Thirty-one patients with surgically and histopathologically confirmed adnexal lesions underwent short echo-time STEAM (stimulated echo acquisition method) 1H- MR spectroscopy, and the results obtained were analysed.
RESULTS
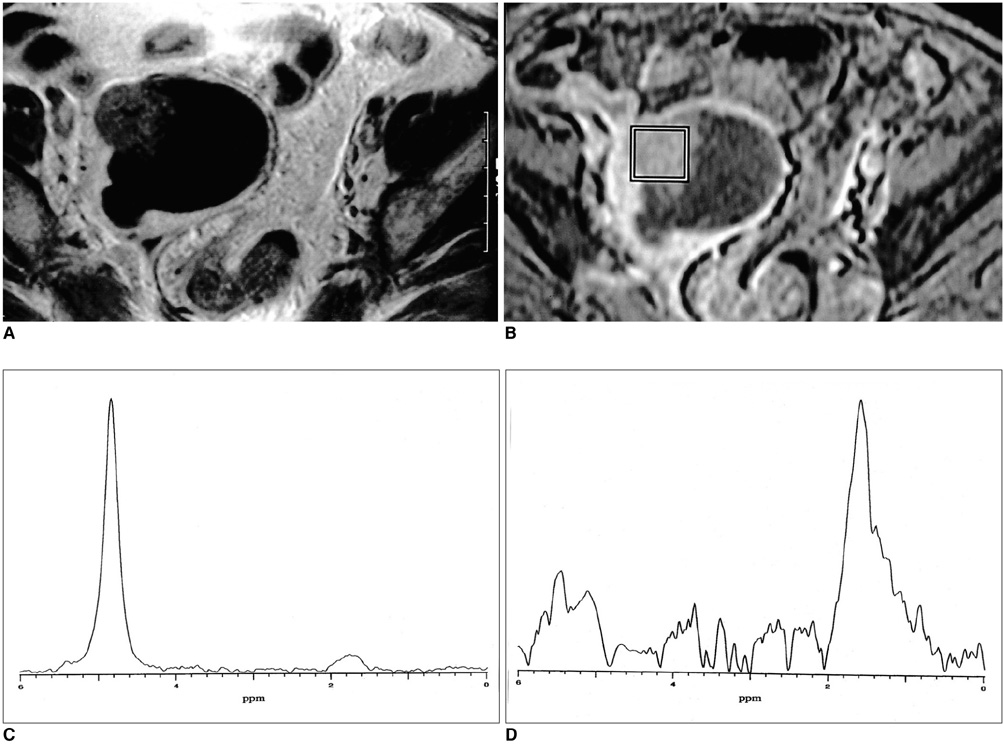
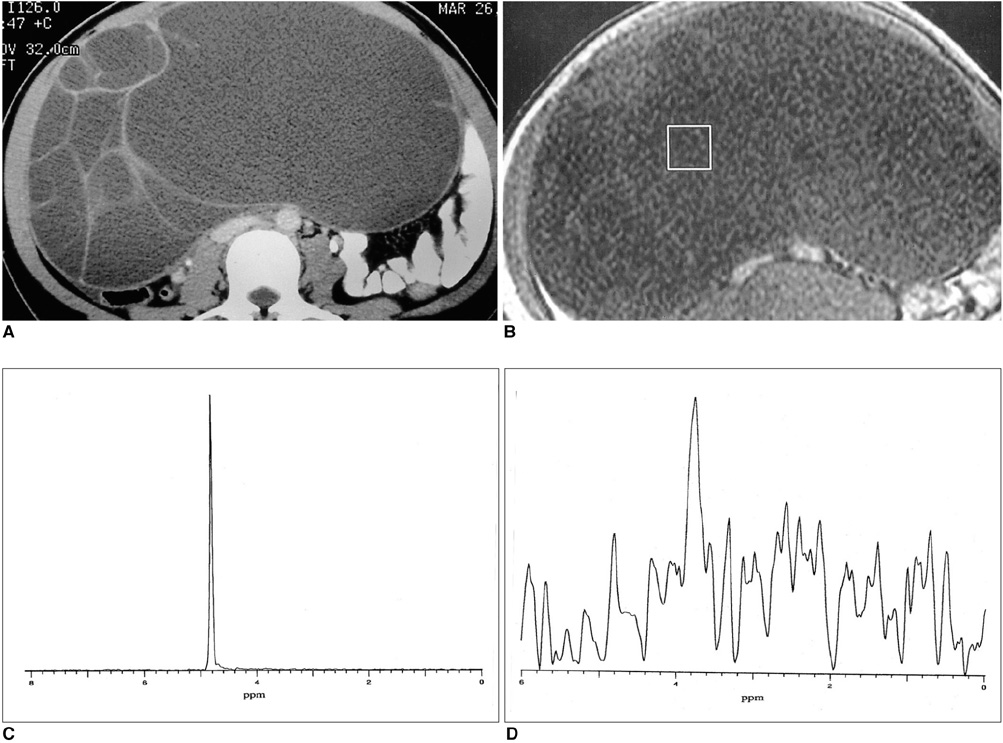
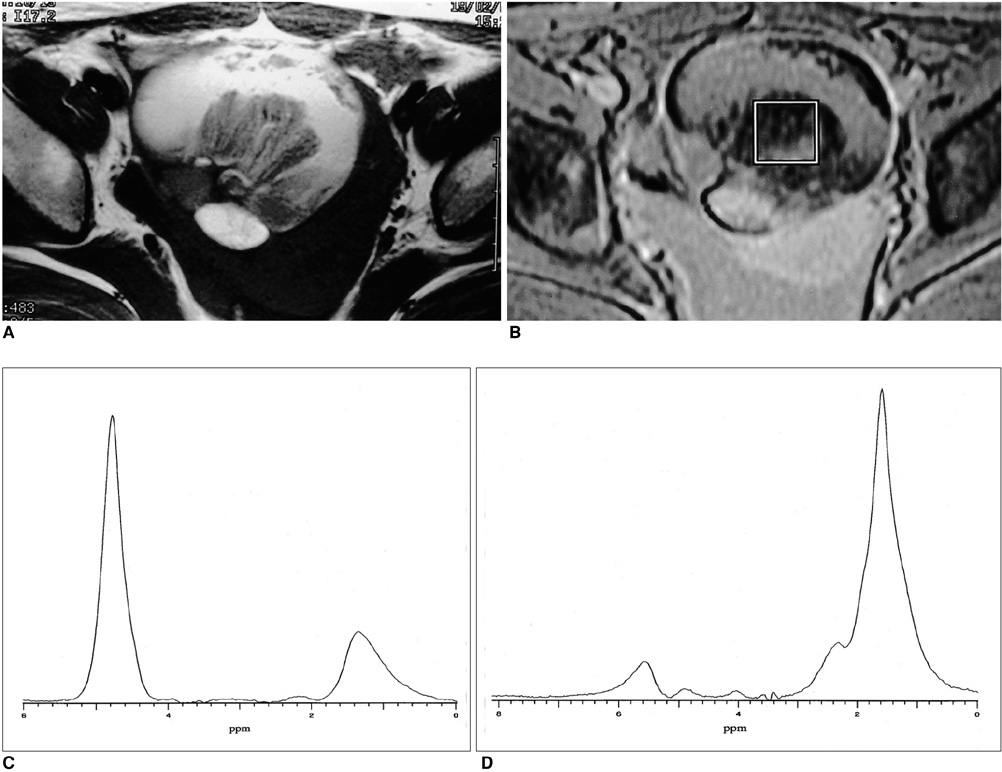
The methylene present in fatty acid chains gave rise to a lipid peak of 1.3 ppm in the 1H- MR spectra of most malignant tumors and benign teratomas. This same peak was not observed, however, in the spectra of benign ovarian epithelial tumors: in a number of these, a peak of 5.2 ppm, due to the presence of the olefine group (-CH=CH-) was noted. The ratios of lipid peak at 1.3 ppm to water peak (lipid/water ratios) varied between disease groups, and in some benign teratomas was characteristically high.
CONCLUSION
An intense lipid peak at 1.3 ppm is observed in malignant ovarian tumors but not in benign epithelial tumors. 1H- MRS may therefore be helpful in the differential diagnosis of adnexal lesions.
MeSH Terms
Figure
Reference
-
1. Granberg S, Norstrom A, Wikland M. Tumors in the lower pelvis as imaged by vaginal sonography. Gynecol Oncol. 1990. 37:224–229.2. Levine D, Feldstein VA, Babcook CJ, Filly RA. Sonography of ovarian masses: poor sensitivity of resistive index for identifying malignant lesions. AJR. 1994. 162:1355–1359.3. Carlson KJ, Skates SJ, Singer DE. Screening for ovarian cancer. Ann Intern Med. 1994. 121:124–132.4. Buist MR, Golding RP, Burger CW, et al. Comparative evaluation of diagnostic methods in ovarian carcinoma, with emphasis on CT and MRI. Gynecol Oncol. 1994. 52:191–198.5. Stevens SK, Hricak H, Stern JL. Ovarian lesions: detection and characterization with Gadolinium-enhanced MR imaging at 1.5 T. Radiology. 1991. 181:481–448.6. Hermann UJ, Locher GW, Goldhirsch A. Sonographic patterns of ovarian tumors: prediction of malignancy. Obstet Gynecol. 1987. 69:777–781.7. Choi C-G, Lee HK, Yoon J-H. Localized proton MR spectroscopic detection of nonketotic hyperglycemia in an infant. Korean J Radiol. 2001. 2:239–242.8. Chang K-H, Kim HD, Park S-W, et al. Usefulness of single voxel proton MR spectroscopy in the evaluation of hippocampal sclerosis. Korean J Radiol. 2000. 1:25–32.9. Kim SH, Chang K-H, Song IC, et al. Brain abscess and brain tumors: discrimination with in-vivo H-1 MR spectroscopy. Radiology. 1997. 204:239–245.10. Lim MK, Suh CH, Kim HJ, et al. Systemic lupus erythematosus: brain MR imaging and single-voxel hydrogen 1 MR spectroscopy. Radiology. 2000. 217:43–49.11. Massuger LF, van Vierzen PB, Engelke U, Heerschap A, Wevers R. 1H-magnetic resonance spectroscopy: a new technique to discriminate benign from malignant ovarian tumors. Cancer. 1998. 82(9):1726–1730.12. Kuesel AC, Briere KM, Halliday WC, Sutherland GR, Donnelly SM, Smith ICP. Mobile lipid accumulation in necrotic tissue of high-grade astrocytomas. Anticancer Research. 1996. 16:1485–1490.13. Lee JH, Cho KS, Kim Y-M, et al. Localized in-vivo 1H nuclear MR spectroscopy for evaluation of human uterine cervical carcinoma. AJR. 1998. 170:1279–1282.14. Gotsis ED, Fountas K, Kapsalaki E, Toulas P, Peristeris G, Papadakis N. In-vivo proton MR spectroscopy: the diagnostic possibilities of lipid resonances in brain tumors. Anticancer Research. 1996. 16:1565–1568.15. Behar KL, Rothman DL, Spencer DD, Petroff OAC. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994. 32:294–302.16. Hwang J-H, Graham GD, Behar KL, Alger JR, Prichard JW, Rothman DL. Short echo time proton magnetic resonance spectroscopic imaging of macromolecule and metabolic signal intensities in the human brain. Magn Reson Med. 1996. 35:633–639.17. Posse S, Schuknecht B, Smith ME, van Zijl PCM, Herschkovitz N, Moonen CTW. Short echo time proton MR spectroscopic imaging. J Comput Assist Tomogr. 1994. 17:1–14.18. Hwang J-H, Egnaczyk GF, Ballard E, Dunn RS, Holland SK, Ball WS Jr. Proton MR spectroscopic characteristics of pediatric pilocytic astrocytomas. AJNR. 1998. 19:535–540.19. Mountfold CE, Saunders JK, May GL, et al. Classification of human tumors by high-resolution magnetic resonance spectroscopy. Lancet. 1986. 1:651–652.20. Moonen CTW, von Kienlin M, van Ziji PCM, et al. Comparison of single shot localization methos (STEAM and PRESS) for in-vivo proton NMR spectroscopy. NMR Biomed. 1989. 2:201–208.21. Schiebler ML, Miyamoto KK, White M, Maygargen SJ, Mohler JL. In vitro high resolution 1H-spectroscopy of the human prostate: benign prostatic hyperplasia, normal peripheral zone and adenocarcinoma. Magn Reson Med. 1993. 29:285–291.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vivo Proton MR Spectroscopic Findings of Focal Hepatic Lesions: Initial Experience
- Comparison of in Vivo, in Vitro 3T MR Spectroscopy and Proton NMR Spectroscopy for the Fluid from Cystic Tumor: Preliminary Study
- In Vivo Proton Magnetic Resonance Spectroscopic Findings in Brain Abscess: A Case Report
- Use of Nuclear Magnetic Resonance Spectroscopy in Analysis of Fennel Essential Oil
- In-Vivo Proton Magnetic Resonance Spectroscopy of 2-Hydroxyglutarate in Isocitrate Dehydrogenase-Mutated Gliomas: A Technical Review for Neuroradiologists