Endocrinol Metab.
2018 Mar;33(1):62-69. 10.3803/EnM.2018.33.1.62.
Comparison of Immunohistochemistry and Direct Sanger Sequencing for Detection of the BRAF(V600E) Mutation in Thyroid Neoplasm
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. wongukim@amc.seoul.kr
- 2Division of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. hipuha@hanmail.net
- KMID: 2407122
- DOI: http://doi.org/10.3803/EnM.2018.33.1.62
Abstract
- BACKGROUND
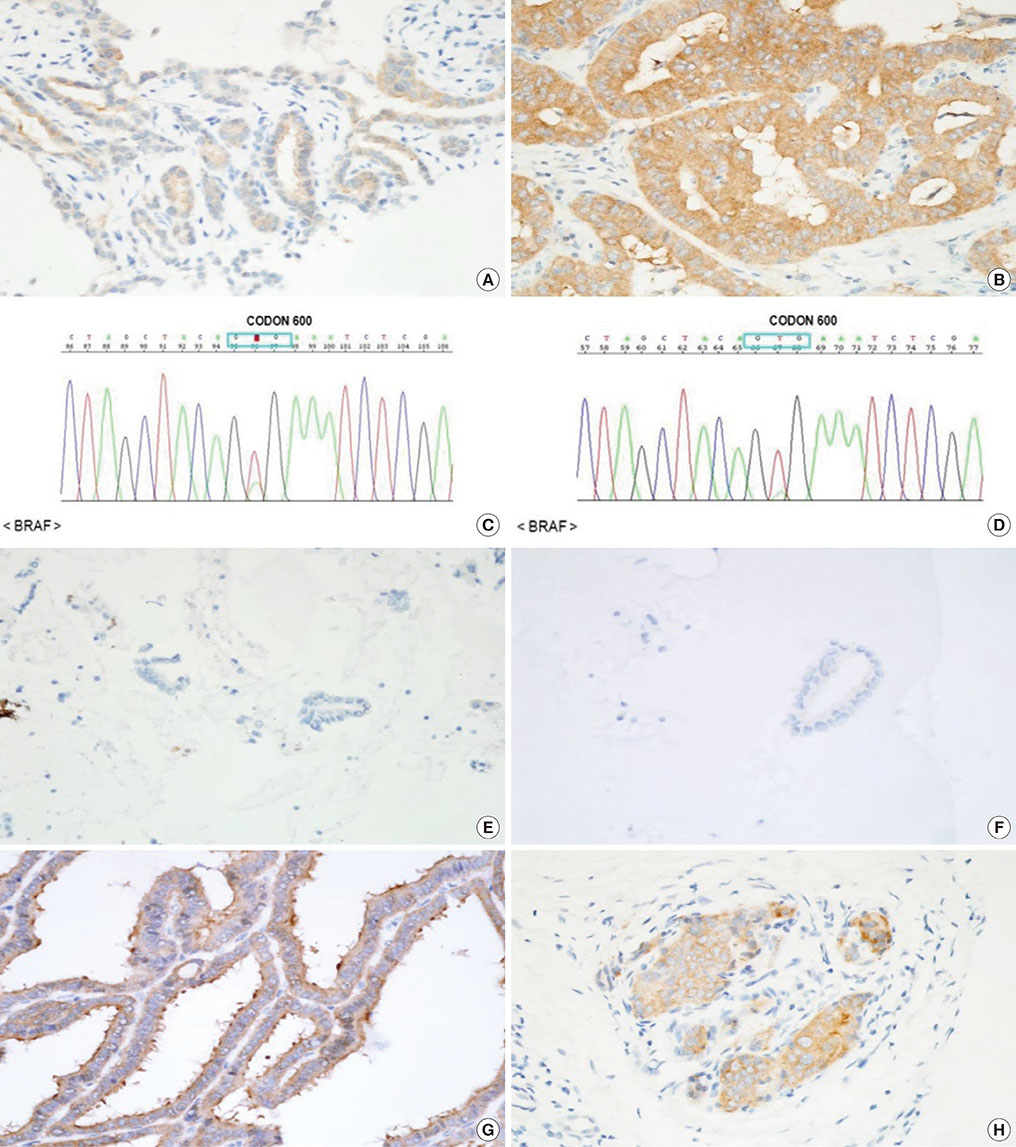
The BRAF V600E mutation is the most common genetic alteration identified in papillary thyroid carcinoma (PTC). Because of its costs effectiveness and sensitivity, direct Sanger sequencing has several limitations. The aim of this study was to evaluate the efficiency of immunohistochemistry (IHC) as an alternative method to detect the BRAF V600E mutation in preoperative and postoperative tissue samples.
METHODS
We evaluated 71 patients who underwent thyroid surgery with the result of direct sequencing of the BRAF V600E mutation. IHC staining of the BRAF V600E mutation was performed in 49 preoperative and 23 postoperative thyroid specimens.
RESULTS
Sixty-two patients (87.3%) had PTC, and of these, BRAF V600E was confirmed by direct sequencing in 57 patients (91.9%). In 23 postoperative tissue samples, the BRAF V600E mutation was detected in 16 samples (70%) by direct sequencing and 18 samples (78%) by IHC. In 24 fine needle aspiration (FNA) samples, BRAF V600E was detected in 18 samples (75%) by direct sequencing and 16 samples (67%) by IHC. In 25 core needle biopsy (CNB) samples, the BRAF V600E mutation was detected in 15 samples (60%) by direct sequencing and 16 samples (64%) by IHC. The sensitivity and specificity of IHC for detecting the BRAF V600E mutation were 77.8% and 66.7% in FNA samples and 99.3% and 80.0% in CNB samples.
CONCLUSION
IHC could be an alternative method to direct Sanger sequencing for BRAF V600E mutation detection both in postoperative and preoperative samples. However, application of IHC to detect the BRAF V600E mutation in FNA samples is of limited value compared with direct sequencing.
MeSH Terms
Figure
Reference
-
1. Kim KH, Kang DW, Kim SH, Seong IO, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med J. 2004; 45:818–821.
Article2. Kim TY, Kim WB, Song JY, Rhee YS, Gong G, Cho YM, et al. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin Endocrinol (Oxf). 2005; 63:588–593.3. Jung CK, Im SY, Kang YJ, Lee H, Jung ES, Kang CS, et al. Mutational patterns and novel mutations of the BRAF gene in a large cohort of Korean patients with papillary thyroid carcinoma. Thyroid. 2012; 22:791–797.
Article4. Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004; 116:855–867.
Article5. Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011; 7:569–580.
Article6. Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine (Baltimore). 2012; 91:274–286.7. Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005; 90:6373–6379.
Article8. Elisei R, Viola D, Torregrossa L, Giannini R, Romei C, Ugolini C, et al. The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab. 2012; 97:4390–4398.9. Choi SH, Baek JH, Lee JH, Choi YJ, Song DE, Chung KW, et al. Evaluation of the clinical usefulness of BRAFV600E mutation analysis of core-needle biopsy specimens in thyroid nodules with previous atypia of undetermined significance or follicular lesions of undetermined significance results. Thyroid. 2015; 25:897–903.
Article10. Borrelli N, Ugolini C, Giannini R, Antonelli A, Giordano M, Sensi E, et al. Role of gene expression profiling in defining indeterminate thyroid nodules in addition to BRAF analysis. Cancer Cytopathol. 2016; 124:340–349.11. Ihle MA, Fassunke J, Konig K, Grunewald I, Schlaak M, Kreuzberg N, et al. Comparison of high resolution melting analysis, pyrosequencing, next generation sequencing and immunohistochemistry to conventional Sanger sequencing for the detection of p.V600E and non-p.V600E BRAF mutations. BMC Cancer. 2014; 14:13.
Article12. Monzon FA, Ogino S, Hammond ME, Halling KC, Bloom KJ, Nikiforova MN. The role of KRAS mutation testing in the management of patients with metastatic colorectal cancer. Arch Pathol Lab Med. 2009; 133:1600–1606.
Article13. Tol J, Dijkstra JR, Vink-Borger ME, Nagtegaal ID, Punt CJ, Van Krieken JH, et al. High sensitivity of both sequencing and real-time PCR analysis of KRAS mutations in colorectal cancer tissue. J Cell Mol Med. 2010; 14:2122–2131.
Article14. Colomba E, Helias-Rodzewicz Z, Von Deimling A, Marin C, Terrones N, Pechaud D, et al. Detection of BRAF p.V600E mutations in melanomas: comparison of four methods argues for sequential use of immunohistochemistry and pyrosequencing. J Mol Diagn. 2013; 15:94–100.15. Carbonell P, Turpin MC, Torres-Moreno D, Molina-Martinez I, Garcia-Solano J, Perez-Guillermo M, et al. Comparison of allelic discrimination by dHPLC, HRM, and TaqMan in the detection of BRAF mutation V600E. J Mol Diagn. 2011; 13:467–473.
Article16. Jiang W, Wang W, Fu F, Teng X, Wang H, Wang H, et al. A more sensitive platform for the detection of low-abundance BRAF(V600E) mutations. Mol Cell Biochem. 2012; 366:49–58.
Article17. Routhier CA, Mochel MC, Lynch K, Dias-Santagata D, Louis DN, Hoang MP. Comparison of 2 monoclonal antibodies for immunohistochemical detection of BRAF V600E mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Hum Pathol. 2013; 44:2563–2570.
Article18. Dvorak K, Aggeler B, Palting J, McKelvie P, Ruszkiewicz A, Waring P. Immunohistochemistry with the anti-BRAF V600E (VE1) antibody: impact of pre-analytical conditions and concordance with DNA sequencing in colorectal and papillary thyroid carcinoma. Pathology. 2014; 46:509–517.
Article19. Bullock M, O'Neill C, Chou A, Clarkson A, Dodds T, Toon C, et al. Utilization of a MAB for BRAF(V600E) detection in papillary thyroid carcinoma. Endocr Relat Cancer. 2012; 19:779–784.
Article20. Rossi ED, Martini M, Capodimonti S, Cenci T, Straccia P, Angrisani B, et al. Analysis of immunocytochemical and molecular BRAF expression in thyroid carcinomas: a cytohistologic institutional experience. Cancer Cytopathol. 2014; 122:527–535.
Article21. Cibas ES, Ali SZ. The Bethesda System for reporting thyroid cytopathology. Thyroid. 2009; 19:1159–1165.
Article22. Jung CK, Min HS, Park HJ, Song DE, Kim JH, Park SY, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean endocrine pathology thyroid core needle biopsy study group. J Pathol Transl Med. 2015; 49:288–299.
Article23. DeLellis RA. Pathology and genetics of tumours of endocrine organs. Lyon: IARC Press;2004.24. Oh HS, Kim WG, Park S, Kim M, Kwon H, Jeon MJ, et al. Serial neck ultrasonographic evaluation of changes in papillary thyroid carcinoma during pregnancy. Thyroid. 2017; 27:773–777.
Article25. Kwon H, Kim WG, Eszlinger M, Paschke R, Song DE, Kim M, et al. Molecular diagnosis using residual liquid-based cytology materials for patients with nondiagnostic or indeterminate thyroid nodules. Endocrinol Metab (Seoul). 2016; 31:586–591.
Article26. Jeon MJ, Song DE, Jung CK, Kim WG, Kwon H, Lee YM, et al. Impact of reclassification on thyroid nodules with architectural atypia: from non-invasive encapsulated follicular variant papillary thyroid carcinomas to non-invasive follicular thyroid neoplasm with papillary-like nuclear features. PLoS One. 2016; 11:e0167756.
Article27. Choi SH, Baek JH, Lee JH, Choi YJ, Ha EJ, Song DE, et al. Initial clinical experience with BRAF(V600E) mutation analysis of core-needle biopsy specimens from thyroid nodules. Clin Endocrinol (Oxf). 2016; 84:607–613.28. Koperek O, Kornauth C, Capper D, Berghoff AS, Asari R, Niederle B, et al. Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carcinoma. Am J Surg Pathol. 2012; 36:844–850.
Article29. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.30. Lee SE, Hwang TS, Choi YL, Kim WY, Han HS, Lim SD, et al. Molecular profiling of papillary thyroid carcinoma in Korea with a high prevalence of BRAF(V600E) mutation. Thyroid. 2017; 27:802–810.31. Leslie C, Grieu-Iacopetta F, Richter A, Platten M, Murray J, Frost FA, et al. BRAF p.Val600Glu (V600E) mutation detection in thyroid fine needle aspiration cell block samples: a feasibility study. Pathology. 2015; 47:432–438.
Article32. Wobker SE, Kim LT, Hackman TG, Dodd LG. Use of BRAF v600e immunocytochemistry on FNA direct smears of papillary thyroid carcinoma. Cancer Cytopathol. 2015; 123:531–539.
Article33. Crescenzi A, Guidobaldi L, Nasrollah N, Taccogna S, Cicciarella Modica DD, Turrini L, et al. Immunohistochemistry for BRAF(V600E) antibody VE1 performed in core needle biopsy samples identifies mutated papillary thyroid cancers. Horm Metab Res. 2014; 46:370–374.
Article34. Capper D, Preusser M, Habel A, Sahm F, Ackermann U, Schindler G, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011; 122:11–19.
Article35. Martinuzzi C, Pastorino L, Andreotti V, Garuti A, Minuto M, Fiocca R, et al. A combination of immunohistochemistry and molecular approaches improves highly sensitive detection of BRAF mutations in papillary thyroid cancer. Endocrine. 2016; 53:672–680.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Implication of BRAF Mutation in Thyroid Cancer
- Clinicopathological Implications of the BRAF(V600E) Mutation in PTC with Concurrent Hashimoto Thyroiditis
- Association of BRAF(V600E) Mutation with Poor Clinical Prognostic Factors and Ultrasonographic Findings in Cases of Papillary Thyroid Carcinoma
- Evaluation of the Anyplex BRAF V600E Real-Time Detection Assay Using Dual-Priming Oligonucleotide Technology in Fine-Needle Aspirates of Thyroid Nodules
- Expressions of miRNAs in Papillary Thyroid Carcinoma and Their Associations with the BRAFV600EMutation and Clinicopathological Features.