Kosin Med J.
2020 Jun;35(1):1-14. 10.7180/kmj.2020.35.1.1.
Expressions of miRNAs in Papillary Thyroid Carcinoma and Their Associations with the BRAFV600EMutation and Clinicopathological Features.
- Affiliations
-
- 1Department of Surgery, College of Medicine, Kosin University, Busan, Korea
- 2Department of Internal Medicine, College of Medicine, Kosin University, Busan, Korea
- KMID: 2503724
- DOI: http://doi.org/10.7180/kmj.2020.35.1.1
Abstract
Objectives
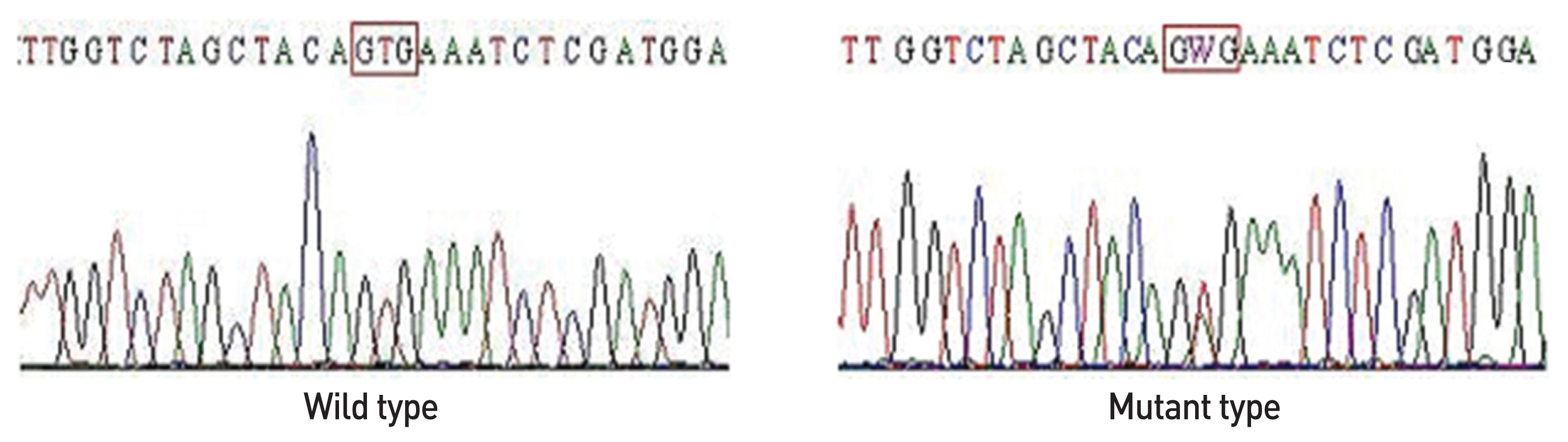
The microRNAs (miRNAs) are known to be commonly expressed in papillary thyroid carcinoma. The BRAFV600Emutation is the most common genetic mutation in thyroid cancer. The main aim of this study was to determine the possible association between expression of the three miRNAs and that of BRAFV600E mutation and the clinicopathological features in papillary thyroid carcinoma.
Methods
This study was conducted on 51 paraffin-embedded tissues (42 thyroid cancer, 9 benign tumor) obtained from patients undergoing thyroidectomy at the Endocrine Center of OOO University Hospital.
Results
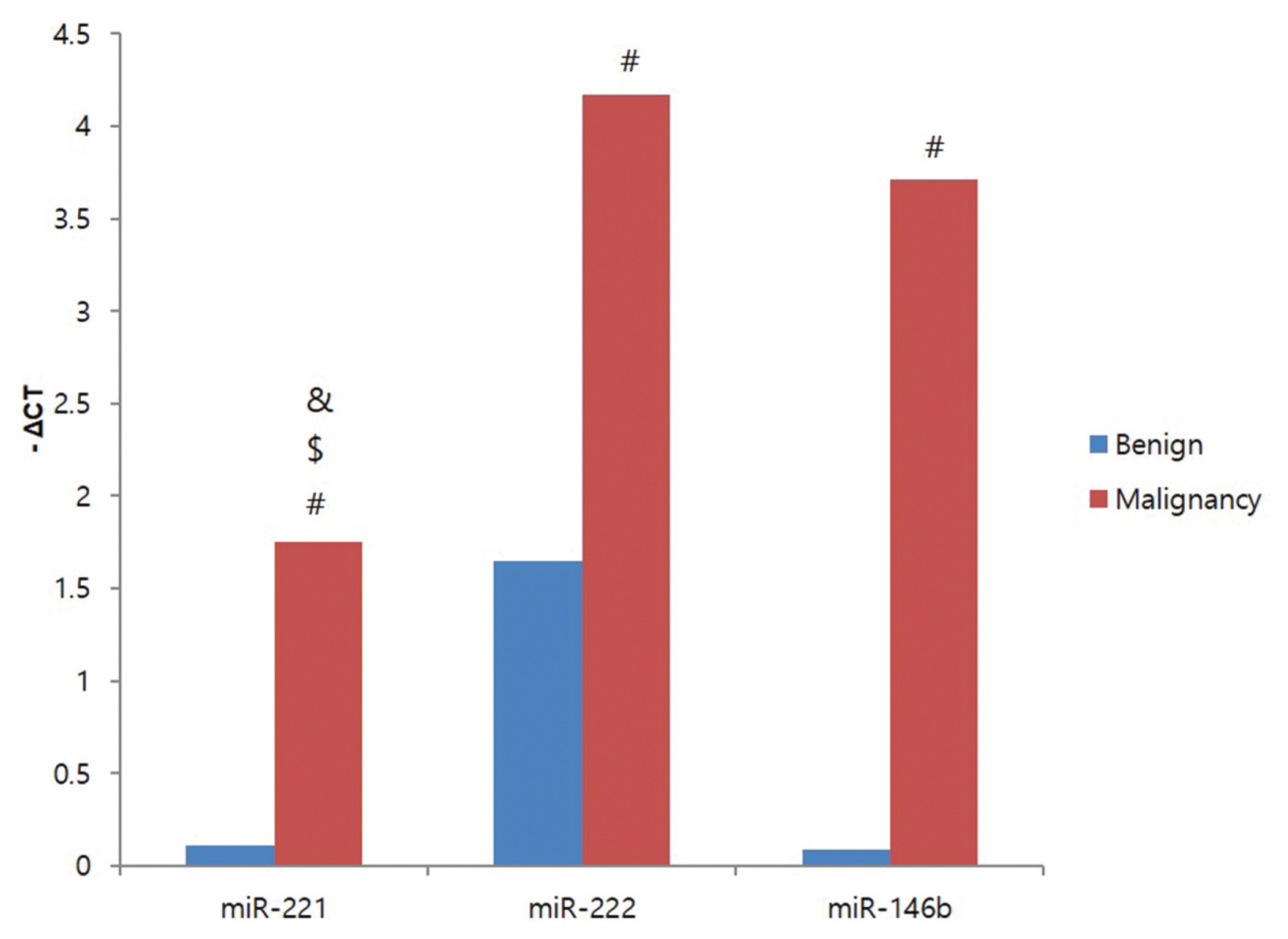
miRNAs expression was significantly high in patients with cervical lymph node metastasis and advanced TNM stage. In addition, miR-146b expression levels were significantly higher in papillary thyroid carcinoma patients with BRAFV600E mutation. The relative quantification (2-△△Ct) of miR-146b was also high among the miRNAs. Individually, the AUCs for miRNA-146b was 0.923 (cutoff value -1.97, sensitivity 88.9%, specificity 85.7%).
Conclusions
Especially, expression of miR-146b increased higher in PTC patients with BRAFV600Emutation. These findings showed a role of miR-146b as potential biomarkers in differentiating PTC from benign tumor and as a prognostic indicator of PTCs. Further investigation will need for the roles of miRNAs in the pathogenesis of papillary thyroid carcinomas.
Keyword
Figure
Reference
-
1. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006; 295:2164–7.
Article2. Ruegemer JJ, Hay ID, Bergstralh EJ, Ryan JJ, Offord KP, Gorman CA. Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 1988; 67:501–8.
Article3. Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid carcinoma. J Clin Endocrinol Metab. 2005; 90:6373–9.4. Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH, Duh QY, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007; 246:466–70.
Article5. Kim SW, Lee JI, Kim JW, Ki CS, Oh YL, Choi YL, et al. BRAFV600E mutation analysis in fine-needle aspiration cytology specimens for evaluation of thyroid nodule: a large series in a BRAFV600E-prevalent population. J Clin Endocrinol Metab. 2010; 95:3693–700.6. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007; 28:742–62.
Article7. Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003; 88:4393–7.8. Ito Y, Yoshida H, Maruo R, Morita S, Takano T, Hirokawa M, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J. 2009; 56:89–97.
Article9. Zeng Y. Principles of micro-RNA production and maturation. Oncogene. 2006; 25:6156–62.
Article10. Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001; 293:1146–50.
Article11. Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006; 6:259–69.12. Dalmay T, Edwards DR. MicroRNAs and the hallmarks of cancer. Oncogene. 2006; 25:6170–5.
Article13. Chen YT, Kitabayashi N, Zhou XK, Fahey TJ 3rd, Scognamiglio T. MicroRNA analysis as a potential diagnostic tool for papillary thyroid carcinoma. Mod Pathol. 2008; 21:1139–46.
Article14. He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci U S A. 2005; 102:19075–80.
Article15. Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008; 93:1600–8.
Article16. Cahill S, Smyth P, Denning K, Flavin R, Li J, Potratz A, et al. Effect of BRAFV600E mutation on transcription and post-transcriptional regulation in a papillary thyroid carcinoma model. Mol Cancer. 2007; 6:21–30.17. Chou CK, Chen RF, Chou FF, Chang HW, Chen YJ, Lee YF, et al. miR-146b is highly expressed in adult papillary thyroid carcinomas with high risk features including extrathyroidal invasion and the BRAFV600E mutation. Thyroid. 2010; 20:489–94.18. Chou CK, Yang KD, Chou FF, Huang CC, Lan YW, Lee YF, et al. Prognostic implications of miR-146b expression and its functional role in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013; 98:196–205.
Article19. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed. New York (NY): Springer;2016.20. Sun Y, Yu S, Liu Y, Wang F, Liu Y, Xiao H. Expression of miRNAs in Papillary Thyroid Carcinomas Is Associated with BRAF Mutation and Clinicopathological Features in Chinese Patients. Int J Endocrinol. 2013; 128:735.21. Nga ME, Kumarasinghe MP, Tie B, Sterrett GF, Wood B, Walsh J, et al. Experience with standardized thyroid fine needle aspiration reporting categories: follow-up data from 529 cases with ‘indeterminate’ or ‘atypical’ reports. Cancer Cytopathol. 2010; 118:423–33.22. Geraldo MV, Yamashita AS, Kimura ET. MicroRNA miR-146b-5p regulates signal transduction of TGF-β by repressing SMAD4 in thyroid cancer. Oncogene. 2012; 31:1910–22.
Article23. Garcia AI, Buisson M, Bertrand P, Rimokh R, Rouleau E, Lopez BS, et al. Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. EMBO Mol Med. 2011; 3:279–90.24. Scapoli L, Palmieri A, Lo Muzio L, Pezzetti F, Rubini C, Girardi A, et al. microRNA expression profiling of oral carcinoma identifies new markers of tumor progression. Int J Immunopathol Pharmacol. 2010; 23:1229–34.
Article25. Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005; 65:4238–45.
Article26. Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005; 115:1068–81.
Article27. Salvatore G, DeFalco V, Salerno P, Nappi TC, Pepe S, Troncone G, et al. BRAF is a therapeutic target in aggressive thyroid carcinoma. Clin Cancer Res. 2006; 12:1623–9.
Article28. Yip L, Kelly L, Shuai Y, Armstrong MJ, Nikiforov YE, Carty SE, et al. MicroRNA Signature Distinguishes the Degree of Aggressiveness of Papillary Thyroid Carcinoma. Ann Surg Oncol. 2011; 18:2035–41.
Article29. Sheu SY, Grabellus F, Schwertheim S, Handke S, Worm K, Schmid KW. Lack of correlation between BRAF V600E mutational status and the expression profile of a distinct set of miRNAs in papillary thyroid carcinoma. Horm Metab Res. 2009; 41:482–7.30. Huang Y, Liao D, Pan L, Ye R, Li X, Wang S, et al. Expressions of miRNAs in papillary thyroid carcinoma and their associations with the BRAFV600E mutation. Eur J Endocrinol. 2013; 15. 168:675–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association between BRAF(V600E) Mutations and Clinicopathological Features of Papillary Thyroid Microcarcinoma (PTMC)
- Clinicopathological Implications of the BRAF(V600E) Mutation in PTC with Concurrent Hashimoto Thyroiditis
- Association of BRAF(V600E) Mutation with Poor Clinical Prognostic Factors and Ultrasonographic Findings in Cases of Papillary Thyroid Carcinoma
- A Case of Multifocal Papillary Thyroid Carcinoma Consisting of One Encapsulated Follicular Variant with BRAF K601E Mutation and Three Conventional Types with BRAF V600E Mutation
- HIF-1alpha Expression in BRAF(V600E)-Positive Papillary Thyroid Microcarcinoma