Endocrinol Metab.
2015 Mar;30(1):98-104. 10.3803/EnM.2015.30.1.98.
Enhancement of Short-Term Memory by Methyl-6-(Phenylethynyl)-Pyridine in the BTBR T+tf/J Mouse Model of Autism Spectrum Disorder
- Affiliations
-
- 1Department of Pharmacology, Hallym University College of Medicine, Chuncheon, Korea.
- 2Department of Physical Education, Hallym University College of Natural Science, Chuncheon, Korea. jayshong@hallym.ac.kr
- KMID: 2407111
- DOI: http://doi.org/10.3803/EnM.2015.30.1.98
Abstract
- BACKGROUND
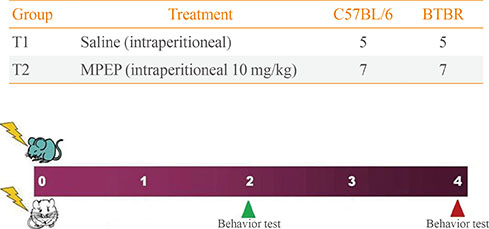
Autism spectrum disorder (ASD) encompasses a range of disorders that are characterized by social and communication deficits and repetitive behaviors. This study evaluated the effect of methyl-6-(phenylethynyl)-pyridine (MPEP), an antagonist of the mGluR5 metabotropic glutamate receptor, on memory enhancement in the BTBR T+tf/J (BTBR) mouse strain, which has been recognized as a model of ASD.
METHODS
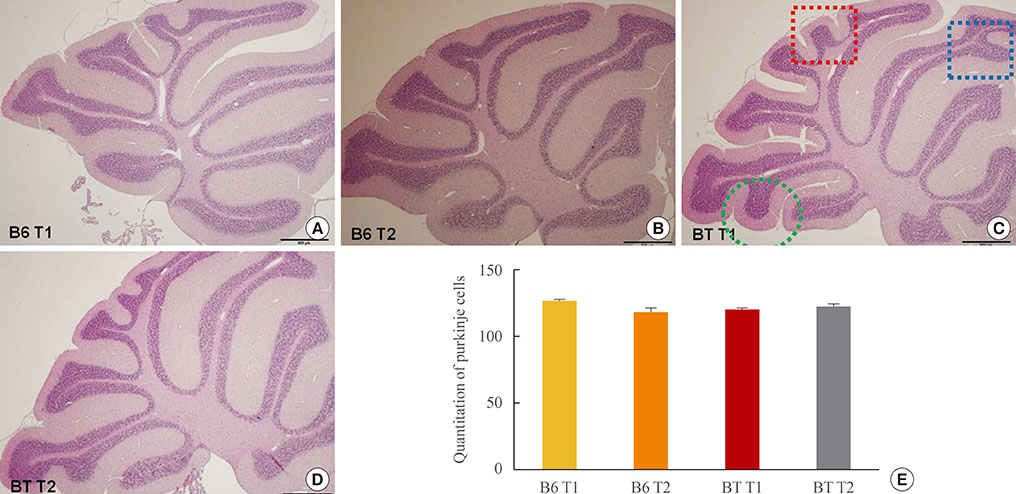
The pharmacological effects of MPEP on memory and motor coordination were assessed using the Morris water maze and rotarod tests in BTBR and C57BL/6J (B6) mice. Furthermore, we performed morphological analyses of cerebellar foliation in BTBR and B6 mice using hematoxylin and eosin staining.
RESULTS
MPEP-treated BTBR mice exhibited improved learning and memory in the Morris water maze test. MPEP administration also improved motor coordination in the rotarod test. However, no significant difference was observed regarding the numbers of Purkinje cells in the cerebella of BTBR versus normal B6 mice.
CONCLUSION
This study suggests that the mGluR5 antagonist MPEP has the potential to ameliorate learning and memory dysfunction and impaired motor coordination in BTBR mice. These results further suggest that the BTBR mouse model may be useful in pharmacological studies investigating drugs that could potentially alleviate cognitive dysfunction in ASD.
Keyword
MeSH Terms
Figure
Reference
-
1. Manning-Courtney P, Murray D, Currans K, Johnson H, Bing N, Kroeger-Geoppinger K, Sorensen R, Bass J, Reinhold J, Johnson A, Messerschmidt T. Autism spectrum disorders. Curr Probl Pediatr Adolesc Health Care. 2013; 43:2–11.2. Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000; 28:355–363.3. Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001; 2:943–955.4. Ronald A, Happe F, Bolton P, Butcher LM, Price TS, Wheelwright S, Baron-Cohen S, Plomin R. Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry. 2006; 45:691–699.5. Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. Am J Psychiatry. 1995; 152:1145–1149.6. Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Arch Neurol. 1995; 52:794–801.7. Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010; 35:976–989.8. Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008; 13:4–26.9. Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007; 176:4–20.10. Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007; 1:1.11. Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008; 3:e3067.12. Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007; 176:21–26.13. Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol. 2007; 17:172–179.14. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47–60.15. Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996; 86:159–171.16. Barnes CA, Danysz W, Parsons CG. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. Eur J Neurosci. 1996; 8:565–571.17. Blokland A, Geraerts E, Been M. A detailed analysis of rats' spatial memory in a probe trial of a Morris task. Behav Brain Res. 2004; 154:71–75.18. Tarantino LM, Gould TJ, Druhan JP, Bucan M. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mamm Genome. 2000; 11:555–564.19. Brandon EP, Logue SF, Adams MR, Qi M, Sullivan SP, Matsumoto AM, Dorsa DM, Wehner JM, McKnight GS, Idzerda RL. Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice. J Neurosci. 1998; 18:3639–3649.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the Female Estrous Cycle on the Sexual Behaviors and Ultrasonic Vocalizations of Male C57BL/6 and Autistic BTBR T+ tf/J Mice
- The Translation of the Term 'Autism Spectrum Disorder' in Korean
- Exploring the Validity of Valproic Acid Animal Model of Autism
- Screening Instruments for Autism Spectrum Disorder: Mini Review
- Comparison of the Autism Diagnostic Observation Schedule and Childhood Autism Rating Scale in the Diagnosis of Autism Spectrum Disorder: A Preliminary Study