Endocrinol Metab.
2015 Mar;30(1):58-64. 10.3803/EnM.2015.30.1.58.
Increased Sclerostin Levels after Further Ablation of Remnant Estrogen by Aromatase Inhibitors
- Affiliations
-
- 1Department of Internal Medicine, Endocrine Research Institute, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. YUMIE@yuhs.ac
- 2The Graduated School Yonsei University, Seoul, Korea.
- 3Department of Internal Medicine, Kwandong University College of Medicine, Incheon, Korea.
- 4Department of General Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2407105
- DOI: http://doi.org/10.3803/EnM.2015.30.1.58
Abstract
- BACKGROUND
Sclerostin is a secreted Wnt inhibitor produced almost exclusively by osteocytes, which inhibits bone formation. Aromatase inhibitors (AIs), which reduce the conversion of steroids to estrogen, are used to treat endocrine-responsive breast cancer. As AIs lower estrogen levels, they increase bone turnover and lower bone mass. We analyzed changes in serum sclerostin levels in Korean women with breast cancer who were treated with an AI.
METHODS
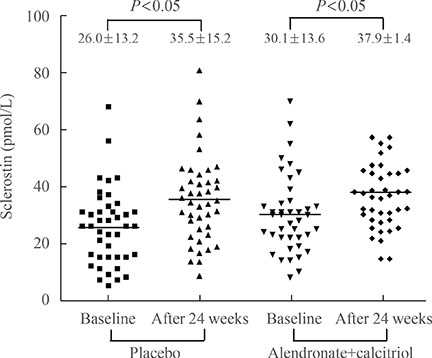
We included postmenopausal women with endocrine-responsive breast cancer (n=90; mean age, 57.7 years) treated with an AI, and compared them to healthy premenopausal women (n=36; mean age, 28.0 years). The subjects were randomly assigned to take either 5 mg alendronate with 0.5 microg calcitriol (n=46), or placebo (n=44) for 6 months.
RESULTS
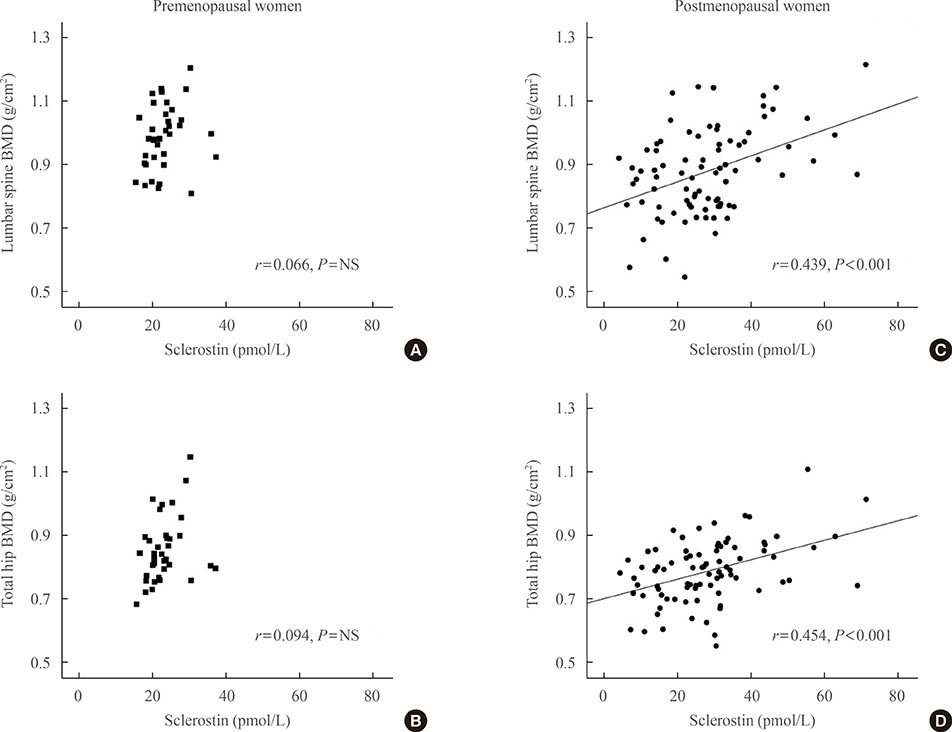
Postmenopausal women with breast cancer had significantly higher sclerostin levels compared to those in premenopausal women (27.8+/-13.6 pmol/L vs. 23.1+/-4.8 pmol/L, P<0.05). Baseline sclerostin levels positively correlated with either lumbar spine or total hip bone mineral density only in postmenopausal women (r=0.218 and r=0.233; P<0.05, respectively). Serum sclerostin levels increased by 39.9%+/-10.2% 6 months after AI use in postmenopausal women; however, no difference was observed between the alendronate and placebo groups (39.9%+/-10.2% vs. 55.9%+/-9.13%, P>0.05).
CONCLUSION
Serum sclerostin levels increased with absolute deficiency of residual estrogens in postmenopausal women with endocrine-responsive breast cancer who underwent AI therapy with concurrent bone loss.
MeSH Terms
Figure
Cited by 1 articles
-
Osteoblasts Are the Centerpiece of the Metastatic Bone Microenvironment
Hyo Min Jeong, Sun Wook Cho, Serk In Park
Endocrinol Metab. 2016;31(4):485-492. doi: 10.3803/EnM.2016.31.4.485.
Reference
-
1. Parfitt AM. The bone remodeling compartment: a circulatory function for bone lining cells. J Bone Miner Res. 2001; 16:1583–1585.2. Rhee Y, Song K, Park S, Park HS, Lim SK, Park BW. Efficacy of a combined alendronate and calcitriol agent (Maxmarvil®) in Korean postmenopausal women with early breast cancer receiving aromatase inhibitor: a double-blind, randomized, placebo-controlled study. Endocr J. 2013; 60:167–172.3. Kneissel M. The promise of sclerostin inhibition for the treatment of osteoporosis. IBMS Bonekey. 2009; 6:259–264.4. Moester MJ, Papapoulos SE, Lowik CW, van Bezooijen RL. Sclerostin: current knowledge and future perspectives. Calcif Tissue Int. 2010; 87:99–107.5. Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003; 22:6267–6276.6. Mirza FS, Padhi ID, Raisz LG, Lorenzo JA. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab. 2010; 95:1991–1997.7. Modder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res. 2011; 26:27–34.8. Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010; 95:5056–5062.9. Galea GL, Sunters A, Meakin LB, Zaman G, Sugiyama T, Lanyon LE, Price JS. Sost down-regulation by mechanical strain in human osteoblastic cells involves PGE2 signaling via EP4. FEBS Lett. 2011; 585:2450–2454.10. Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Jahanzeb M, Kiel K, Ljung BM, Marcom PK, Mayer IA, McCormick B, Nabell LM, Pierce LJ, Reed EC, Smith ML, Somlo G, Theriault RL, Topham NS, Ward JH, Winer EP, Wolff AC. NCCN Breast Cancer Clinical Practice Guidelines Panel. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009; 7:122–192.11. Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS. ATAC Trialists' Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005; 365:60–62.12. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005; 97:1262–1271.13. Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C. Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004; 350:1081–1092.14. Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008; 26:1051–1057.15. Brufsky A, Bundred N, Coleman R, Lambert-Falls R, Mena R, Hadji P, Jin L, Schenk N, Ericson S, Perez EA. Z-FAST and ZO-FAST Study Groups. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008; 13:503–514.16. Brufsky A, Harker WG, Beck JT, Carroll R, Tan-Chiu E, Seidler C, Hohneker J, Lacerna L, Petrone S, Perez EA. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007; 25:829–836.17. Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009; 24:1651–1661.18. Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008; 283:5866–5875.19. Robling AG, Bellido T, Turner CH. Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact. 2006; 6:354.20. Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ 3rd, Khosla S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011; 26:373–379.21. Polyzos SA, Anastasilakis AD, Bratengeier C, Woloszczuk W, Papatheodorou A, Terpos E. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women: the six-month effect of risedronate and teriparatide. Osteoporos Int. 2012; 23:1171–1176.22. Arasu A, Cawthon PM, Lui LY, Do TP, Arora PS, Cauley JA, Ensrud KE, Cummings SR. Study of Osteoporotic Fractures Research Group. Serum sclerostin and risk of hip fracture in older Caucasian women. J Clin Endocrinol Metab. 2012; 97:2027–2032.23. Garnero P, Sornay-Rendu E, Munoz F, Borel O, Chapurlat RD. Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int. 2013; 24:489–494.24. Ardawi MS, Rouzi AA, Al-Sibiani SA, Al-Senani NS, Qari MH, Mousa SA. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: the Center of Excellence for Osteoporosis Research Study. J Bone Miner Res. 2012; 27:2592–2602.25. Sapir-Koren R, Livshits G. Is interaction between age-dependent decline in mechanical stimulation and osteocyte-estrogen receptor levels the culprit for postmenopausal-impaired bone formation? Osteoporos Int. 2013; 24:1771–1789.26. Rossini M, Gatti D, Adami S. Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013; 93:121–132.27. Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009; 24:578–588.28. Li X, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Grisanti M, Dwyer D, Stouch B, Thway TM, Stolina M, Ominsky MS, Kostenuik PJ, Simonet WS, Paszty C, Ke HZ. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res. 2010; 25:2647–2656.29. Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, Graham K, Tipton B, Cai J, Deshpande R, Zhou L, Hale MD, Lightwood DJ, Henry AJ, Popplewell AG, Moore AR, Robinson MK, Lacey DL, Simonet WS, Paszty C. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res. 2010; 25:948–959.30. Chung YE, Lee SH, Lee SY, Kim SY, Kim HH, Mirza FS, Lee SK, Lorenzo JA, Kim GS, Koh JM. Long-term treatment with raloxifene, but not bisphosphonates, reduces circulating sclerostin levels in postmenopausal women. Osteoporos Int. 2012; 23:1235–1243.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pattern Alopecia during Hormonal Anticancer Therapy in Patients with Breast Cancer
- Endocrine Therapy for Breast Cancer
- Survival Benefit of Zoledronic Acid in Postmenopausal Breast Cancer Patients Receiving Aromatase Inhibitors
- Romosozumab for the treatment of osteoporosis
- Elevated Circulating Sclerostin Levels in Frail Older Adults: Implications beyond Bone Health