Blood Res.
2017 Dec;52(4):300-306. 10.5045/br.2017.52.4.300.
The limited role of serum galactomannan assay in screening for invasive pulmonary aspergillosis in allogeneic stem cell transplantation recipients on micafungin prophylaxis: a retrospective study
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University, College of Medicine, Seoul, Korea. wbpark1@snu.ac.kr, ihkimmd@snu.ac.kr
- 2Cancer Research Institute, Seoul National University, College of Medicine, Seoul, Korea.
- KMID: 2405067
- DOI: http://doi.org/10.5045/br.2017.52.4.300
Abstract
- BACKGROUND
We evaluated the outcomes of serum galactomannan (GM) assay for the screening of invasive pulmonary aspergillosis (IPA) in allogeneic hematopoietic stem cell transplantation (alloHSCT) recipients while on primary antifungal prophylaxis (PAP).
METHODS
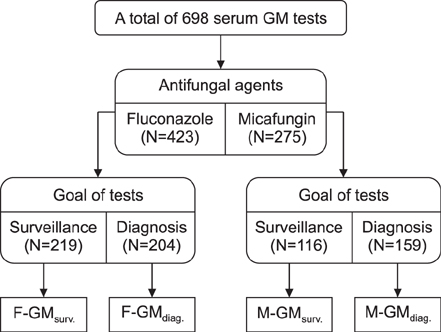
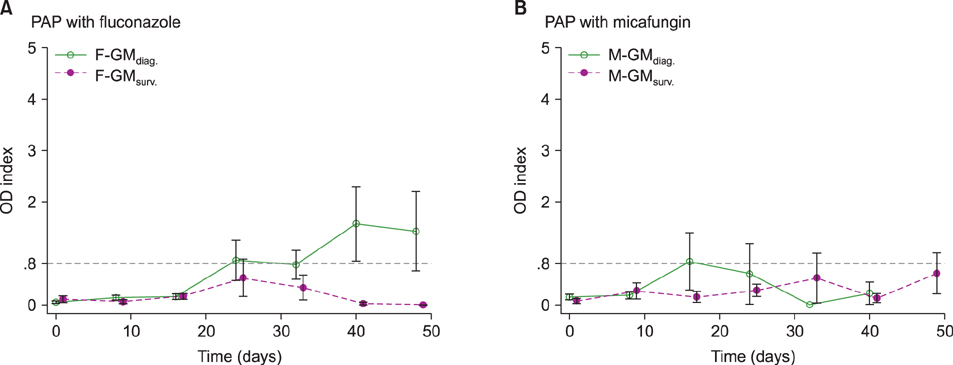
This study included patients with hematologic disorders who underwent alloHSCT from January 2013 to November 2015. Patients received routine PAP with fluconazole before 2014 and micafungin after 2014; serum GM tests were performed and retrospectively analyzed. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of serum GM tests for detection of probable/proven IPA were evaluated. The serial change of serum GM levels was illustrated on a time series plot.
RESULTS
A total of 136 alloHSCT recipients at Seoul National University Hospital were included in the study. Fluconazole was administered in 72 patients for PAP, while micafungin was administered in the remaining 64 patients. The overall sensitivity, specificity, and NPV of serum GM assays were 95.8% (95% confidence interval [CI] 78.9-99.9%), 93.8% (95% CI 91.7-95.5%), and 99.8% (95% CI 99.1-100.0%), respectively. However, the PPV of GM tests was relatively low at 35.4% (95% CI 23.9-48.2%). The serial change in serum GM levels differed according to the antifungal agents used. With effective PAP using micafungin, serial serum GM levels showed zero order kinetics during the neutropenic period.
CONCLUSION
Although the serum GM assay is a sensitive and specific test for detecting IPA in alloHSCT recipients, its role for routine surveillance in an era of effective PAP with micafungin is limited.
Keyword
MeSH Terms
Figure
Reference
-
1. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007; 356:348–359.
Article2. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007; 25:5471–5489.
Article3. Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007; 356:335–347.
Article4. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007; 44:531–540.
Article5. Latgé JP, Kobayashi H, Debeaupuis JP, et al. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect Immun. 1994; 62:5424–5433.
Article6. Cuenca-Estrella M, Bassetti M, Lass-Flörl C, Rácil Z, Richardson M, Rogers TR. Detection and investigation of invasive mould disease. J Antimicrob Chemother. 2011; 66:Suppl 1. i15–i24.
Article7. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008; 46:1813–1821.
Article8. Marchetti O, Lamoth F, Mikulska M, Viscoli C, Verweij P, Bretagne S. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant. 2012; 47:846–854.
Article9. Boutboul F, Alberti C, Leblanc T, et al. Invasive aspergillosis in allogeneic stem cell transplant recipients: increasing antigenemia is associated with progressive disease. Clin Infect Dis. 2002; 34:939–943.
Article10. Maertens J, Verhaegen J, Lagrou K, Van Eldere J, Boogaerts M. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood. 2001; 97:1604–1610.
Article11. Hashino S, Morita L, Takahata M, et al. Administration of micafungin as prophylactic antifungal therapy in patients undergoing allogeneic stem cell transplantation. Int J Hematol. 2008; 87:91–97.
Article12. Hiramatsu Y, Maeda Y, Fujii N, et al. Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol. 2008; 88:588–595.
Article13. Hirata Y, Yokote T, Kobayashi K, et al. Antifungal prophylaxis with micafungin in neutropenic patients with hematological malignancies. Leuk Lymphoma. 2010; 51:853–859.
Article14. Sirohi B, Powles RL, Chopra R, et al. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. 2006; 38:47–51.
Article15. van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004; 39:1407–1416.
Article16. Borlenghi E, Cattaneo C, Capucci MA, et al. Usefulness of the MSG/IFICG/EORTC diagnostic criteria of invasive pulmonary aspergillosis in the clinical management of patients with acute leukaemia developing pulmonary infiltrates. Ann Hematol. 2007; 86:205–210.
Article17. Subirà M, Martino R, Rovira M, Vazquez L, Serrano D, De La Cámara R. Clinical applicability of the new EORTC/MSG classification for invasive pulmonary aspergillosis in patients with hematological malignancies and autopsy-confirmed invasive aspergillosis. Ann Hematol. 2003; 82:80–82.
Article18. Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005; 16:73–81.
Article19. Ambasta A, Carson J, Church DL. The use of biomarkers and molecular methods for the earlier diagnosis of invasive aspergillosis in immunocompromised patients. Med Mycol. 2015; 53:531–557.
Article20. Asano-Mori Y, Kanda Y, Oshima K, et al. False-positive Aspergillus galactomannan antigenaemia after haematopoietic stem cell transplantation. J Antimicrob Chemother. 2008; 61:411–416.
Article21. Aubry A, Porcher R, Bottero J, et al. Occurrence and kinetics of false-positive Aspergillus galactomannan test results following treatment with beta-lactam antibiotics in patients with hematological disorders. J Clin Microbiol. 2006; 44:389–394.
Article22. Cordonnier C, Botterel F, Ben Amor R, et al. Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis. Clin Microbiol Infect. 2009; 15:81–86.
Article23. Marr KA, Laverdiere M, Gugel A, et al. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005; 40:1762–1769.
Article24. Mennink-Kersten MA, Klont RR, Warris A, Op den Camp HJ, Verweij PE. Bifidobacterium lipoteichoic acid and false ELISA reactivity in aspergillus antigen detection. Lancet. 2004; 363:325–327.
Article25. Racil Z, Kocmanova I, Lengerova M, Winterova J, Mayer J. Intravenous PLASMA-LYTE as a major cause of false-positive results of platelia Aspergillus test for galactomannan detection in serum. J Clin Microbiol. 2007; 45:3141–3142.
Article26. Sulahian A, Touratier S, Ribaud P. False positive test for aspergillus antigenemia related to concomitant administration of piperacillin and tazobactam. N Engl J Med. 2003; 349:2366–2367.
Article27. Surmont I, Stockman W. Gluconate-containing intravenous solutions: another cause of false-positive galactomannan assay reactivity. J Clin Microbiol. 2007; 45:1373.
Article28. Donnelly JP, Leeflang MM. Galactomannan detection and diagnosis of invasive aspergillosis. Clin Infect Dis. 2010; 50:1070–1071.
Article29. Reid MC, Lachs MS, Feinstein AR. Use of methodological standards in diagnostic test research. Getting better but still not good. JAMA. 1995; 274:645–651.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Micafungin for Prophylaxis of Invasive Fungal Infection in Hematopoietic Stem Cell Transplantation Recipients
- Disseminated Aspergillosis following Allogeneic Hematopoietic Stem Cell Transplantation in an Acute Leukemic Patient who was Previously Treated for Invasive Aspergillosis
- A Case of Invasive Pulmonary Aspergillosis Cured with Liposomal Amphotericin, Granulocyte Transfusion and Surgery in an Allogeneic Hemopoietic Stem Cell Transplantation Recipient
- Successful Allogeneic Hematopoietic Stem Cell Transplantation for a Patient with Very Severe Aplastic Anemia During Active Invasive Fungal Infection
- Impact of previous invasive pulmonary aspergillosis on the outcome of allogeneic hematopoietic stem cell transplantation