Ann Lab Med.
2018 Mar;38(2):85-94. 10.3343/alm.2018.38.2.85.
Multi-center Performance Evaluations of Tacrolimus and Cyclosporine Electrochemiluminescence Immunoassays in the Asia-Pacific Region
- Affiliations
-
- 1Peking Union Medical College Hospital, Beijing, China.
- 2Department of Pharmacology, Jinling Hospital, Medical School of Nanjing University, Nanjing, China.
- 3The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
- 4Tianjin First Center Hospital, Tianjin, China.
- 5Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Pathology, Hospital Kuala Lumpur Drug and Research Laboratory, Kuala Lumpur, Malaysia.
- 7Roche Diagnostics, Indianapolis, USA.
- 8Roche Diagnostics, Penzberg, Germany.
- 9Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sailchun@amc.seoul.kr
- KMID: 2403352
- DOI: http://doi.org/10.3343/alm.2018.38.2.85
Abstract
- BACKGROUND
The immunosuppressant drugs (ISDs), tacrolimus and cyclosporine, are vital for solid organ transplant patients to prevent rejection. However, toxicity is a concern, and absorption is highly variable across patients; therefore, ISD levels need to be precisely monitored. In the Asia-Pacific (APAC) region, tacrolimus and cyclosporine concentrations are typically measured using immunoassays. The objective of this study was to assess the analytical performance of Roche Elecsystacrolimus and cyclosporinee electrochemiluminescence immunoassays (ECLIAs).
METHODS
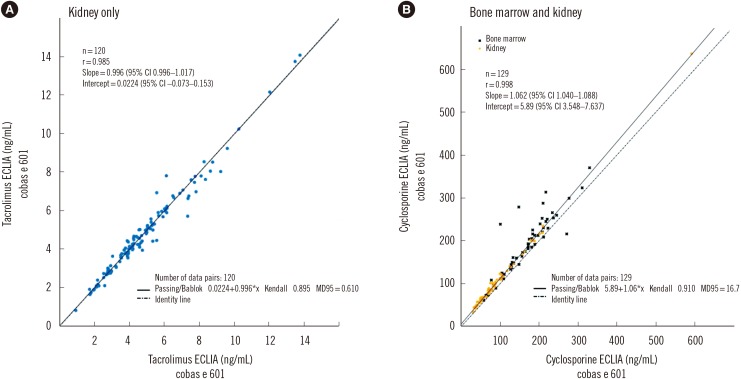
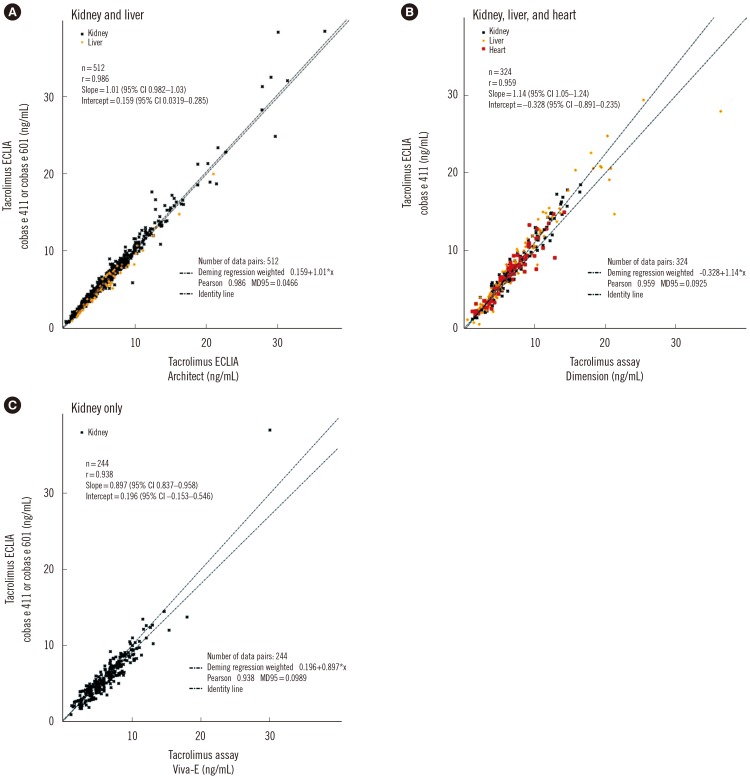
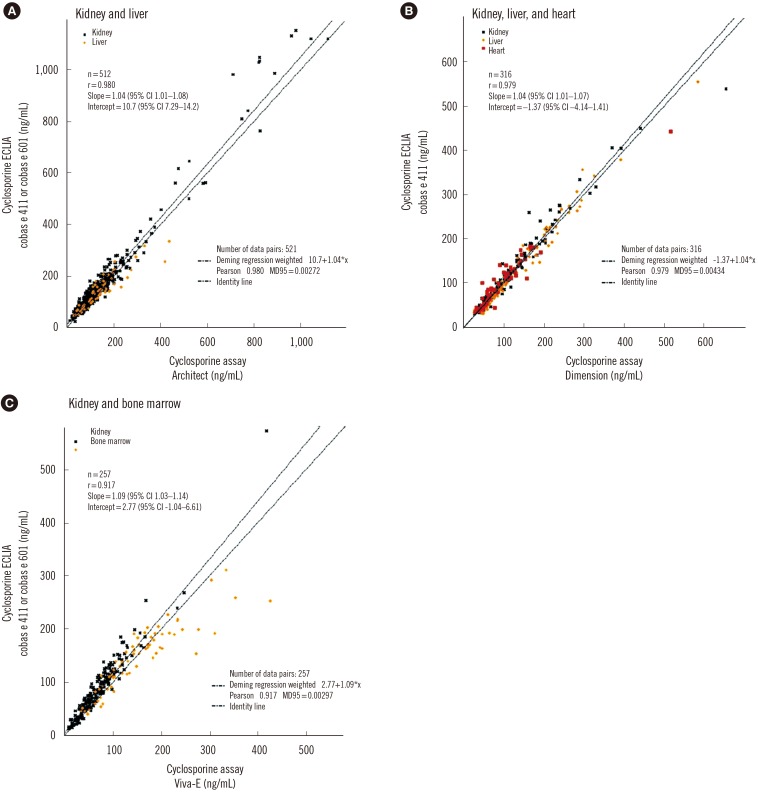
This evaluation was performed in seven centers across China, South Korea, and Malaysia. Imprecision (repeatability and reproducibility), assay accuracy, and lot-to-lot reagent variability were tested. The Elecsys ECLIAs were compared with commercially available immunoassays (Architect, Dimension, and Viva-E systems) using whole blood samples from patients with various transplant types (kidney, liver, heart, and bone marrow).
RESULTS
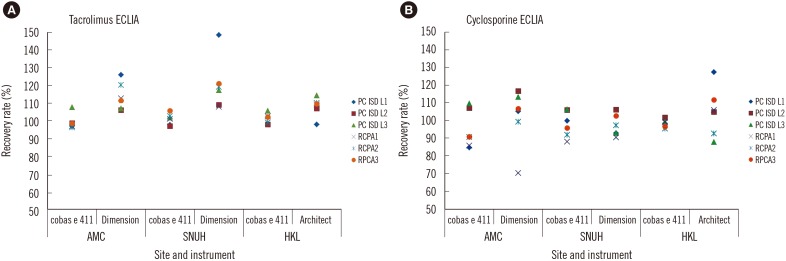
Coefficients of variation for repeatability and reproducibility were ≤5.4% and ≤12.4%, respectively, for the tacrolimus ECLIA, and ≤5.1% and ≤7.3%, respectively, for the cyclosporine ECLIA. Method comparisons of the tacrolimus ECLIA with Architect, Dimension, and Viva-E systems yielded slope values of 1.01, 1.14, and 0.897, respectively. The cyclosporine ECLIA showed even closer agreements with the Architect, Dimension, and Viva-E systems (slope values of 1.04, 1.04, and 1.09, respectively). No major differences were observed among the different transplant types.
CONCLUSIONS
The tacrolimus and cyclosporine ECLIAs demonstrated excellent precision and close agreement with other immunoassays tested. These results show that both assays are suitable for ISD monitoring in an APAC population across a range of different transplant types.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Vancomycin and Aminoglycoside Antibiotic Drug Concentration Measurement: Current Status in Clinical Laboratories in Korea
Won Kyung Kwon, Jongwon Oh, Soo-Youn Lee, Hyung-Doo Park
Lab Med Online. 2020;10(4):265-275. doi: 10.47429/lmo.2020.10.4.265.
Reference
-
1. Khurana A, Brennan DC. Current concepts of immunosuppression and side effects. In : Liapis H, Wang HL, editors. Pathology of solid organ transplantation. Berlin Heidelberg: Springer;2011.2. Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006; (4):CD005161. PMID: 17054241.3. Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2005; (4):CD003961. PMID: 16235347.4. Khan S, Khan S, Baboota S, Ali J. Immunosuppressive drug therapy--biopharmaceutical challenges and remedies. Expert Opin Drug Deliv. 2015; 12:1333–1349. PMID: 25642742.5. Holt DW, Armstrong VW, Griesmacher A, Morris RG, Napoli KL, Shaw LM, et al. International Federation of Clinical Chemistry/International Association of Therapeutic Drug Monitoring and Clinical Toxicology working group on immunosuppressive drug monitoring. Ther Drug Monit. 2002; 24:59–67. PMID: 11805724.6. Kang JS, Lee MH. Overview of therapeutic drug monitoring. Korean J Intern Med. 2009; 24:1–10. PMID: 19270474.7. Shipkova M, Vogeser M, Ramos PA, Verstraete AG, Orth M, Schneider C, et al. Multi-center analytical evaluation of a novel automated tacrolimus immunoassay. Clin Biochem. 2014; 47:1069–1077. PMID: 24721684.8. Vogeser M, Shipkova M, Rigo-Bonnin R, Wallemacq P, Orth M, Widmann M, et al. Multicenter analytical evaluation of the automated electrochemiluminescence immunoassay for cyclosporine. Ther Drug Monit. 2014; 36:640–650. PMID: 24646730.9. Xie F, Ding X, Zhang QY. An update on the role of intestinal cytochrome P450 enzymes in drug disposition. Acta Pharm Sin B. 2016; 6:374–383. PMID: 27709006.10. Zhou HH, Liu ZQ. Ethnic differences in drug metabolism. Clin Chem Lab Med. 2000; 38:899–903. PMID: 11097347.11. Jovanovik M, Bogojeska A, Trajanov D, Kocarev L. Inferring cuisine--drug interactions using the linked data approach. Sci Rep. 2015; 5:9346. PMID: 25792182.12. Seger C, Shipkova M, Christians U, Billaud EM, Wang P, Holt DW, et al. Assuring the proper analytical performance of measurement procedures for immunosuppressive drug concentrations in clinical practice: recommendations of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology Immunosuppressive Drug Scientific Committee. Ther Drug Monit. 2016; 38:170–189. PMID: 26982493.13. Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009; 31:139–152. PMID: 19177031.14. Volosov A, Napoli KL, Soldin SJ. Simultaneous simple and fast quantification of three major immunosuppressants by liquid chromatography--tandem mass-spectrometry. Clin Biochem. 2001; 34:285–290. PMID: 11440728.15. Wu AH, French D. Implementation of liquid chromatography/mass spectrometry into the clinical laboratory. Clin Chim Acta. 2013; 420:4–10. PMID: 23085380.16. Gounden V, Soldin SJ. Tacrolimus measurement: building a better immunoassay. Clin Chem. 2014; 60:575–576. PMID: 24415738.17. Roche Diagnostics. Cyclosporine: method sheet. Accessed on 02 Nov 2016. https://pim-eservices.roche.com.18. Roche Diagnostics. Tacrolimus: method sheet. Accessed on 02 Nov 2016. https://pim-eservices.roche.com.19. Abbott. Architect system: Cyclosporine. 2010. Accessed on 10 Apr 2017. http://www.ilexmedical.com/files/PDF/Cyclosporin_ARC.pdf.20. Abbott. Architect system: Tacrolimus. 2009. Accessed on 10 Apr 2017. http://www.ilexmedical.com/files/PDF/Tacrolimus_ARC.pdf.21. Siemens Healthcare Diagnostics Inc. Dimension Cyclosporine (CSA) and Cyclosporine Extended Range (CSAE) Assays. 2008. Accessed on 10 Apr 2017. https://static.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/groups/public/@global/@clinicalspec/documents/download/mdaw/mtcx/~edisp/0701146_dimension_csa_ss_us-00036452.pdf.22. Siemens Healthcare Diagnostics Inc. Emit® 2000 Tacrolimus (TACR) Assay. 2008. Accessed on 10 Apr 2017. https://static.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/groups/public/@global/@clinicalspec/documents/download/mdaw/mtcx/~edisp/0701146_dimension_csa_ss_us-00036452.pdf.23. Siemens Healthcare Diagnostics Inc. Emit® 2000 Cyclosporine A (CsA) Assay. 2008. Accessed on 10 Apr 2017. https://static.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/groups/public/@global/@clinicalspec/documents/download/mdaw/mtmy/~edisp/0701087_syva_emit_csa_ss_ous-00038804.pdf.24. Royal College of Pathologists of Australasia. Accessed on 02 Nov 2016. https://www.rcpaqap.com.au/.25. Clinical and Laboratory Standards Institute. Evaluation of precision of quantitative measurement methods: approved guideline - third edition: EP05-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2014.26. Clinical and Laboratory Standards Institute. Measurement procedure comparison and bias estimation using patient samples; approved guideline - third edition: EP09-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2013.27. Serdarevic N, Zunic L. Comparison of Architect I 2000 for determination of cyclosporine with AxSYM. Acta Inform Med. 2012; 20:214–217. PMID: 23378685.28. Wallemacq P, Maine GT, Berg K, Rosiere T, Marquet P, Aimo G, et al. Multisite analytical evaluation of the Abbott ARCHITECT cyclosporine assay. Ther Drug Monit. 2010; 32:145–151. PMID: 20216110.29. Chavan P, Bhat V, Gosavi U, Pillai B. Cyclosporine drug levels: comparison of the Architect 1000i with the Siemens Dimension RXL system. J Lab Physicians. 2015; 7:137. PMID: 26417169.30. Amann S, Parker TS, Levine DM. Evaluation of 2 immunoassays for monitoring low blood levels of tacrolimus. Ther Drug Monit. 2009; 31:273–276. PMID: 19142177.31. Jorga A, Holt DW, Johnston A. Therapeutic drug monitoring of cyclosporine. Transplant Proc. 2004; 36:396S–403S. PMID: 15041374.32. Vučeljić M, Lalić N, Mijušković Z, Pejović J. Comparison of two commercial cyclosporin assays. J Med Biochem. 2007; 26:33–37.33. Ko DH, Jeong TD, Lee W, Chun S, Min WK. Performance evaluation of a restored Dimension TACR assay: an automated platform for measuring the whole blood tacrolimus concentration. Clin Lab. 2016; 62:7–12. PMID: 27012028.34. Chung HS, Lee ST, Lee SY. Evaluation of Viva-E drug testing system. Korean J Lab Med. 2007; 27:330–337. PMID: 18094597.35. Holt DW, Mandelbrot DA, Tortorici MA, Korth-Bradley JM, Sierka D, Levy DI, et al. Long-term evaluation of analytical methods used in sirolimus therapeutic drug monitoring. Clin Transplant. 2014; 28:243–251. PMID: 24476346.36. Morelle J, Wallemacq P, Van Caeneghem O, Goffin E. Clinically unexpected cyclosporine levels using the ACMIA method on the RXL dimension analyser. Nephrol Dial Transplant. 2011; 26:1428–1431. PMID: 21335442.37. Hermida J, Tutor JC. Falsely increased blood tacrolimus concentrations using the ACMIA assay due to circulating endogenous antibodies in a liver transplant recipient: a tentative approach to obtaining reliable results. Ther Drug Monit. 2009; 31:269–272. PMID: 19258927.38. Christians U, Vinks AA, Langman LJ, Clarke W, Wallemacq P, van Gelder T, et al. Impact of laboratory practices on interlaboratory variability in therapeutic drug monitoring of immunosuppressive drugs. Ther Drug Monit. 2015; 37:718–724. PMID: 26291980.39. Yatscoff RW, Rosano TG, Bowers LD. The clinical significance of cyclosporine metabolites. Clin Biochem. 1991; 24:23–35. PMID: 2060129.40. Christians U, Sewing KF. Cyclosporin metabolism in transplant patients. Pharmacol Ther. 1993; 57:291–345. PMID: 8361996.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravenous tacrolimus and cyclosporine induced anaphylaxis: what is next?
- Performance Evaluation of the ARCHITECT i2000 for the Determination of Whole Blood Cyclosporin A and Tacrolimus
- Chronic urticaria treated with tacrolimus
- "Asia Pacific Allergy": A new leap forward
- Allergic diseases in the Asia Pacific: path into the future