Clin Exp Vaccine Res.
2018 Jan;7(1):51-60. 10.7774/cevr.2018.7.1.51.
Immunogenicity of the nanovaccine containing intimin recombinant protein in the BALB/c mice
- Affiliations
-
- 1Department of Genetics and Biotechnology, School of Biological Science, Varamin-Pishva Branch, Islamic Azad University, Varamin, Iran.
- 2Applied Microbiology Research Center, Systems Biology and Poisonings Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran. jafar.amani@gmail.com
- 3Department of Biology, Faculty of Science, Imam Hossain University, Tehran, Iran.
- 4Green Gene Company, Tehran, Iran.
- 5Pharmaceutical Sciences Branch, Pharmaceutical Sciences Research Center, Islamic Azad University (IAUPS), Tehran, Iran.
- KMID: 2402538
- DOI: http://doi.org/10.7774/cevr.2018.7.1.51
Abstract
- PURPOSE
Escherichia coli O157:H7 is one of the most important pathogens which create hemorrhagic colitis and hemolytic uremic syndrome in human. It is one of the most prevalent causes of diarrhea leading to death of many people every year. The first diagnosed gene in the locus of enterocyte effacement pathogenicity island is eae gene. The product of this gene is a binding protein called intimin belonging to the group of external membrane proteins regarded as a good stimulants of the immune system. Chitosan with its lipophilic property is an environmentally friendly agent able to return to the environment.
MATERIALS AND METHODS
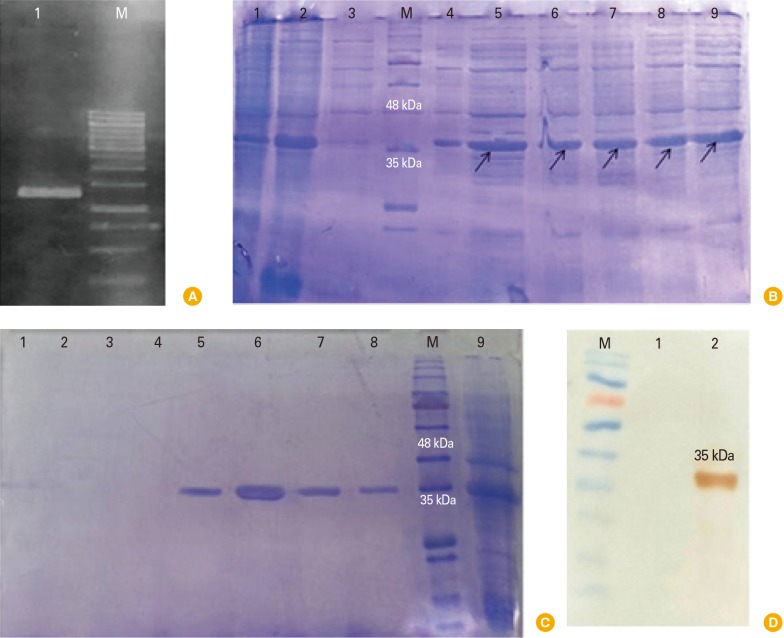
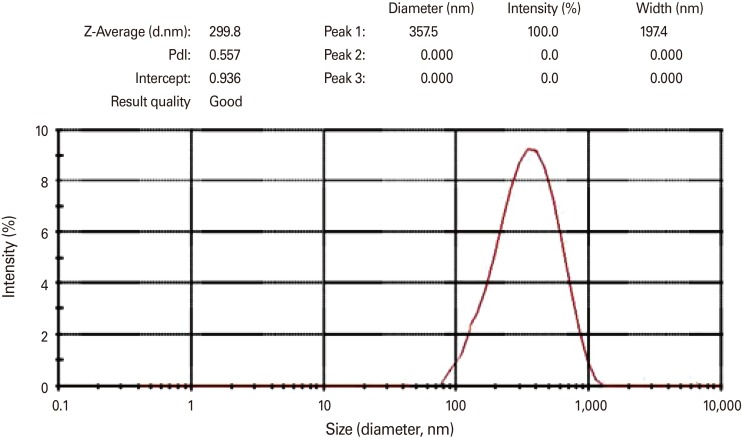
Intimin recombinant protein was expressed in pET28a vector with eae gene and purification was performed using Ni-NTA and finally the recombinant protein was approved through western blotting. This protein was encapsulated using chitosan nanoparticles and the size of nanoparticles was measured by Zetasizer. Intimin encapsulated was prescribed for three sessions among three groups of oral, injection, and oral-injection using Chitosan nanoparticles. Challenge was performed for all three groups with 108 E. coli O157:H7 bacteria.
RESULTS
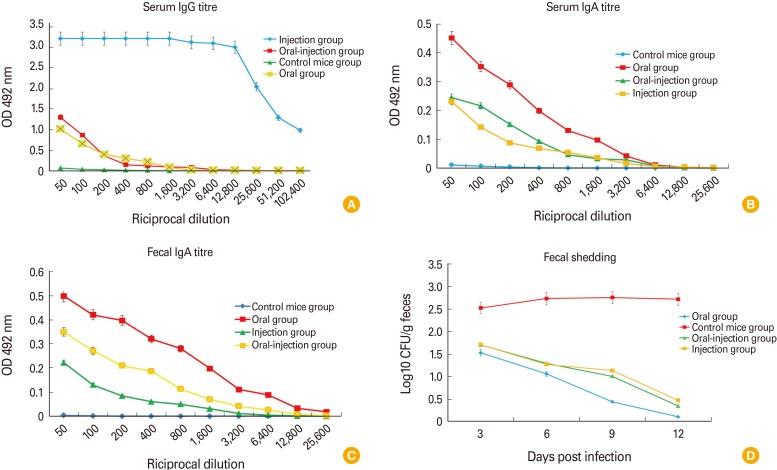
Intimin produced by chitosan nanoparticles improves immunological responses through the adjuvant nature of chitosan nanoparticles. Chitosan may be used as a carrier for transportation of the prescribed vaccine. Among the mice, encapsulated intimin could be able to provide suitable titers of IgG and IgA by the aid of chitosan nanoparticles. Results of mice challenge showed that decreased the bacterial shedding significantly.
CONCLUSION
Results showed that the chitosan nanovaccine with intimin protein may be used as a suitable candidate vaccine against E. coli O157:H7.
MeSH Terms
-
Animals
Bacteria
Bacterial Shedding
Blotting, Western
Carrier Proteins
Chitosan
Colitis
Diarrhea
Enterocytes
Escherichia coli
Genomic Islands
Hemolytic-Uremic Syndrome
Humans
Immune System
Immunoglobulin A
Immunoglobulin G
Membrane Proteins
Mice*
Nanoparticles
Transportation
Carrier Proteins
Chitosan
Immunoglobulin A
Immunoglobulin G
Membrane Proteins
Figure
Reference
-
1. Brooks GF, Jawetz E, Melnick JL, Adelberg EA. Jawetz, Melnick and Adelberg's medical microbiology. New York: McGraw Hill Medical;2010.2. Murray PR, Rosenthal KS, Kobayashi GS, Pfaller MA. Medical microbiology. St. Louis, MO: Mosby;2002. p. 429–441.3. Murphy BP, Murphy M, Buckley JF, et al. In-line milk filter analysis: Escherichia coli O157 surveillance of milk production holdings. Int J Hyg Environ Health. 2005; 208:407–413. PMID: 16217925.
Article4. Blanco JE, Blanco M, Alonso MP, et al. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from human patients: prevalence in Lugo, Spain, from 1992 through 1999. J Clin Microbiol. 2004; 42:311–319. PMID: 14715771.5. te Loo DM, Monnens LA, van Der Velden TJ, et al. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood. 2000; 95:3396–3402. PMID: 10828021.
Article6. Chaisri U, Nagata M, Kurazono H, et al. Localization of Shiga toxins of enterohaemorrhagic Escherichia coli in kidneys of paediatric and geriatric patients with fatal haemolytic uraemic syndrome. Microb Pathog. 2001; 31:59–67. PMID: 11453701.
Article7. Kuhne SA, Hawes WS, La Ragione RM, Woodward MJ, Whitelam GC, Gough KC. Isolation of recombinant antibodies against EspA and intimin of Escherichia coli O157:H7. J Clin Microbiol. 2004; 42:2966–2976. PMID: 15243046.8. Sussman M. Escherichia coli: mechanisms of virulence. New York: Cambridge University Press;1997.9. van Diemen PM, Dziva F, Abu-Median A, et al. Subunit vaccines based on intimin and Efa-1 polypeptides induce humoral immunity in cattle but do not protect against intestinal colonisation by enterohaemorrhagic Escherichia coli O157:H7 or O26:H. Vet Immunol Immunopathol. 2007; 116:47–58. PMID: 17258324.
Article10. Ghaem-Maghami M, Simmons CP, Daniell S, et al. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect Immun. 2001; 69:5597–5605. PMID: 11500434.11. Wales AD, Woodward MJ, Pearson GR. Attaching-effacing bacteria in animals. J Comp Pathol. 2005; 132:1–26. PMID: 15629476.
Article12. Bertin Y, Boukhors K, Livrelli V, Martin C. Localization of the insertion site and pathotype determination of the locus of enterocyte effacement of shiga toxin-producing Escherichia coli strains. Appl Environ Microbiol. 2004; 70:61–68. PMID: 14711626.13. Donnenberg MS, Whittam TS. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J Clin Invest. 2001; 107:539–548. PMID: 11238553.
Article14. Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet. 2004; 363:641–653. PMID: 14987892.
Article15. Krause G, Zimmermann S, Beutin L. Investigation of domestic animals and pets as a reservoir for intimin- (eae) gene positive Escherichia coli types. Vet Microbiol. 2005; 106:87–95. PMID: 15737477.
Article16. Adu-Bobie J, Frankel G, Bain C, et al. Detection of intimins alpha, beta, gamma, and delta, four intimin derivatives expressed by attaching and effacing microbial pathogens. J Clin Microbiol. 1998; 36:662–668. PMID: 9508292.17. Svennerholm AM. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Indian J Med Res. 2011; 133:188–196. PMID: 21415493.18. Fujkuyama Y, Tokuhara D, Kataoka K, et al. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev Vaccines. 2012; 11:367–379. PMID: 22380827.19. Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annu Rev Biomed Eng. 2012; 14:17–46. PMID: 22524387.
Article20. Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002; 6:319–327.
Article21. Cho EJ, Holback H, Liu KC, Abouelmagd SA, Park J, Yeo Y. Nanoparticle characterization: state of the art, challenges, and emerging technologies. Mol Pharm. 2013; 10:2093–2110. PMID: 23461379.
Article22. Bollag DM, Rozycki MD, Edelstein SJ. Protein methods. 2nd ed. New York: Wiley-Liss;1996.23. Aslani MM, Bouzari S. An epidemiological study on Verotoxin-producing Escherichia coli (VTEC) infection among population of northern region of Iran (Mazandaran and Golestan provinces). Eur J Epidemiol. 2003; 18:345–349. PMID: 12803375.
Article24. Potter AA, Klashinsky S, Li Y, et al. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine. 2004; 22:362–369. PMID: 14670317.
Article25. Walker CL, Aryee MJ, Boschi-Pinto C, Black RE. Estimating diarrhea mortality among young children in low and middle income countries. PLoS One. 2012; 7:e29151. PMID: 22235266.
Article26. Shariati Mehr K, Mousavi SL, Rasooli I, Amani J, Rajabi M. A DNA vaccine against Escherichia coli O157:H7. Iran Biomed J. 2012; 16:133–139. PMID: 23023214.27. van Diemen PM, Dziva F, Stevens MP, Wallis TS. Identification of enterohemorrhagic Escherichia coli O26:H- genes required for intestinal colonization in calves. Infect Immun. 2005; 73:1735–1743. PMID: 15731074.28. Dziva F, van Diemen PM, Stevens MP, Smith AJ, Wallis TS. Identification of Escherichia coli O157 : H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology. 2004; 150(Pt 11):3631–3645. PMID: 15528651.29. Cornick NA, Booher SL, Moon HW. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect Immun. 2002; 70:2704–2707. PMID: 11953416.30. Dean-Nystrom EA, Bosworth BT, Moon HW, O'Brien AD. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect Immun. 1998; 66:4560–4563. PMID: 9712821.31. Dean-Nystrom EA, Bosworth BT, Moon HW. Pathogenesis of Escherichia coli O157:H7 in weaned calves. Adv Exp Med Biol. 1999; 473:173–177. PMID: 10659355.
Article32. Paton AW, Manning PA, Woodrow MC, Paton JC. Translocated intimin receptors (Tir) of Shiga-toxigenic Escherichia coli isolates belonging to serogroups O26, O111, and O157 react with sera from patients with hemolytic-uremic syndrome and exhibit marked sequence heterogeneity. Infect Immun. 1998; 66:5580–5586. PMID: 9784578.33. Li Y, Frey E, Mackenzie AM, Finlay BB. Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect Immun. 2000; 68:5090–5095. PMID: 10948130.34. McNeilly TN, Mitchell MC, Corbishley A, et al. Optimizing the protection of cattle against Escherichia coli O157:H7 colonization through immunization with different combinations of H7 flagellin, Tir, Intimin-531 or EspA. PLoS One. 2015; 10:e0128391. PMID: 26020530.
Article35. Amani J, Salmanian AH, Rafati S, Mousavi SL. Immunogenic properties of chimeric protein from espA, eae and tir genes of Escherichia coli O157:H7. Vaccine. 2010; 28:6923–6929. PMID: 20709010.
Article36. Amani J, Mousavi SL, Rafati S, Salmanian AH. Immunogenicity of a plant-derived edible chimeric EspA, Intimin and Tir of Escherichia coli O157:H7 in mice. Plant Sci. 2011; 180:620–627. PMID: 21421410.
Article37. Rabinovitz BC, Larzabal M, Vilte DA, Cataldi A, Mercado EC. The intranasal vaccination of pregnant dams with Intimin and EspB confers protection in neonatal mice from Escherichia coli (EHEC) O157:H7 infection. Vaccine. 2016; 34:2793–2797. PMID: 27129423.
Article38. Chen W, Zhang B, Mahony T, Gu W, Rolfe B, Xu ZP. Efficient and durable vaccine against intimin beta of diarrheagenic E. coli induced by clay nanoparticles. Small. 2016; 12:1627–1639. PMID: 27000499.39. Carroll EC, Jin L, Mori A, et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. 2016; 44:597–608. PMID: 26944200.
Article40. Doavi T, Mousavi SL, Kamali M, Amani J, Fasihi Ramandi M. Chitosan-based intranasal vaccine against Escherichia coli O157:H7. Iran Biomed J. 2016; 20:97–108. PMID: 26724233.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune Response of BALB/c Mice toward Putative Calcium Transporter Recombinant Protein of Trichomonas vaginalis
- Expression of FMD virus-like particles in yeast Hansenula polymorpha and immunogenicity of combine with CpG and aluminum adjuvant
- Protective effects of recombinant Brucella abortus Omp28 against infection with a virulent strain of Brucella abortus 544 in mice
- Immunization of mice with recombinant P27/30 protein confers protection against hard tick Haemaphysalis longicornis (Acari: Ixodidae) infestation
- Immunogenicity of recombinant Lactobacillus plantarum NC8 expressing goose parvovirus VP2 gene in BALB/c mice