Clin Exp Vaccine Res.
2018 Jan;7(1):24-36. 10.7774/cevr.2018.7.1.24.
Calcium-dependent protein kinases are potential targets for Toxoplasma gondii vaccine
- Affiliations
-
- 1Department of Parasitology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran. ghafarif@modares.ac.ir
- KMID: 2402535
- DOI: http://doi.org/10.7774/cevr.2018.7.1.24
Abstract
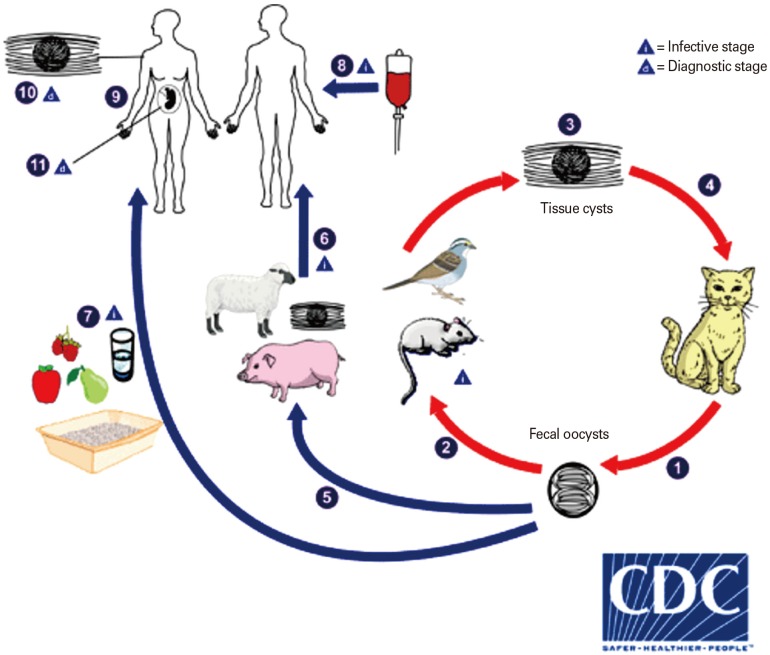
- Toxoplasma gondii belongs to the Apicomplexa phylum that caused a widespread zoonotic infection in wide range of intermediate hosts. Over one-third of the world's population are latently infected with T. gondii and carry it. The complex life cycle of T. gondii indicates the presence of a plurality of antigenic epitopes. During the recent years, continuous efforts of scientists have made precious advances to elucidate the different aspects of the cell and molecular biology of T. gondii. Despite of great progresses, the development of vaccine candidates for preventing of T. gondii infection in men and animals is still remains a challenge. The calcium-dependent protein kinases (CDPKs) belongs to the superfamily of kinases, which restricted to the apicomplexans, ciliates, and plants. It has been documented that they contribute several functions in the life cycle of T. gondii such as gliding motility, cell invasion, and egress as well as some other critical developmental processes. In current paper, we reviewed the recent progress concerning the development of CDPK-based vaccines against acute and chronic T. gondii.
MeSH Terms
Figure
Reference
-
1. Nasiri V, Teymurzadeh S, Karimi G, Nasiri M. Molecular detection of Toxoplasma gondii in snakes. Exp Parasitol. 2016; 169:102–106. PMID: 27522027.
Article2. Dubey JP. The history of Toxoplasma gondii: the first 100 years. J Eukaryot Microbiol. 2008; 55:467–475. PMID: 19120791.3. Foroutan M, Dalvand S, Daryani A, et al. Rolling up the pieces of a puzzle: a systematic review and meta-analysis of the prevalence of toxoplasmosis in Iran. Alex J Med. 2017; 6. 23. –[Epub]. DOI: 10.4132/10.1016/j.ajme.2017.06.003.
Article4. Rostami A, Riahi SM, Fakhri Y, et al. The global seroprevalence of Toxoplasma gondii among wild boars: a systematic review and meta-analysis. Vet Parasitol. 2017; 244:12–20. PMID: 28917302.
Article5. Khademvatan S, Foroutan M, Hazrati-Tappeh K, et al. Toxoplasmosis in rodents: a systematic review and meta-analysis in Iran. J Infect Public Health. 2017; 10:487–493. PMID: 28237696.
Article6. Foroutan M, Majidiani H. Toxoplasma gondii: are there any implications for routine blood screening. Int J Infect. 2017; 10. 31. [Epub]. DOI: 10.5812/iji.62886.
Article7. Foroutan-Rad M, Majidiani H, Dalvand S, et al. Toxoplasmosis in blood donors: a systematic review and meta-analysis. Transfus Med Rev. 2016; 30:116–122. PMID: 27145927.
Article8. Nicolle C, Manceaux L. Sur une infection à corps de Leishman (ou organismes voisins) du gondi. CR Acad Sci. 1908; 147:763–766.9. Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009; 39:1385–1394. PMID: 19433092.
Article10. Wang ZD, Liu HH, Ma ZX, et al. Toxoplasma gondii infection in immunocompromised patients: a systematic review and meta-analysis. Front Microbiol. 2017; 8:389. PMID: 28337191.
Article11. Sullivan WJ Jr, Jeffers V. Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol Rev. 2012; 36:717–733. PMID: 22091606.12. Weiss LM, Dubey JP. Toxoplasmosis: a history of clinical observations. Int J Parasitol. 2009; 39:895–901. PMID: 19217908.
Article13. Mohammadnejad F, Ghaffarifar F, Mobedi I. HIV and parasite co-infection epidemiology: a scope since 2005. Rev Med Microbiol. 2015; 26:20–25.14. Saki J, Khademvatan S, Soltani S, Shahbazian H. Detection of toxoplasmosis in patients with end-stage renal disease by enzyme-linked immunosorbent assay and polymerase chain reaction methods. Parasitol Res. 2013; 112:163–168.
Article15. Yousefi E, Foroutan M, Salehi R, Khademvatan S. Detection of acute and chronic toxoplasmosis amongst multi-transfused thalassemia patients in southwest of Iran. J Acute Dis. 2017; 6:120–125.
Article16. Saki J, Shafieenia S, Foroutan-Rad M. Seroprevalence of toxoplasmosis in diabetic pregnant women in southwestern of Iran. J Parasit Dis. 2016; 40:1586–1589. PMID: 27876989.
Article17. Foroutan-Rad M, Khademvatan S, Majidiani H, Aryamand S, Rahim F, Malehi AS. Seroprevalence of Toxoplasma gondii in the Iranian pregnant women: a systematic review and meta-analysis. Acta Trop. 2016; 158:160–169. PMID: 26952970.18. Abdoli A, Dalimi A, Soltanghoraee H, Ghaffarifar F. Molecular detection and genotypic characterization of Toxoplasma gondii in paraffin-embedded fetoplacental tissues of women with recurrent spontaneous abortion. Int J Fertil Steril. 2017; 10:327–336. PMID: 28042412.19. Fallahi S, Rostami A, Nourollahpour Shiadeh M, Behniafar H, Paktinat S. An updated literature review on maternal-fetal and reproductive disorders of Toxoplasma gondii infection. J Gynecol Obstet Hum Reprod. 2017; 12. 08. [Epub]. DOI: 10.1016/j.jogoh.2017.12.003.
Article20. Centers for Disease Control and Prevention (CDC). Toxoplasmosis [Internet]. Atlanta, GA: Centers for Disease Control and Prevention (CDC);2017. cited 2018 Jan 6. Available from: https://www.cdc.gov/dpdx/toxoplasmosis/.21. Sharif M, Sarvi S, Shokri A, et al. Toxoplasma gondii infection among sheep and goats in Iran: a systematic review and meta-analysis. Parasitol Res. 2015; 114:1–16. PMID: 25378258.
Article22. Majidiani H, Dalvand S, Daryani A, Galvan-Ramirez ML, Foroutan-Rad M. Is chronic toxoplasmosis a risk factor for diabetes mellitus? A systematic review and meta-analysis of case-control studies. Braz J Infect Dis. 2016; 20:605–609. PMID: 27768900.
Article23. Sutterland AL, Fond G, Kuin A, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr Scand. 2015; 132:161–179. PMID: 25877655.24. Flegr J. Neurological and neuropsychiatric consequences of chronic Toxoplasma infection. Curr Clin Microbiol Rep. 2015; 2:163–172.
Article25. Havlicek J, Gasova ZG, Smith AP, Zvara K, Flegr J. Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology. 2001; 122(Pt 5):515–520. PMID: 11393824.
Article26. Webster JP, Brunton CF, MacDonald DW. Effect of Toxoplasma gondii upon neophobic behaviour in wild brown rats, Rattus norvegicus. Parasitology. 1994; 109(Pt 1):37–43. PMID: 8058367.
Article27. Antczak M, Dzitko K, Dlugonska H. Human toxoplasmosis-searching for novel chemotherapeutics. Biomed Pharmacother. 2016; 82:677–684. PMID: 27470411.
Article28. Hiszczynska-Sawicka E, Gatkowska JM, Grzybowski MM, Dlugonska H. Veterinary vaccines against toxoplasmosis. Parasitology. 2014; 141:1365–1378. PMID: 24805159.
Article29. Innes EA. Vaccination against Toxoplasma gondii: an increasing priority for collaborative research? Expert Rev Vaccines. 2010; 9:1117–1119. PMID: 20923261.30. Kur J, Holec-Gasior L, Hiszczynska-Sawicka E. Current status of toxoplasmosis vaccine development. Expert Rev Vaccines. 2009; 8:791–808. PMID: 19485758.
Article31. Garcia JL. Vaccination concepts against Toxoplasma gondii. Expert Rev Vaccines. 2009; 8:215–225. PMID: 19196201.32. Lim SS, Othman RY. Recent advances in Toxoplasma gondii immunotherapeutics. Korean J Parasitol. 2014; 52:581–593. PMID: 25548409.
Article33. Zhang NZ, Chen J, Wang M, Petersen E, Zhu XQ. Vaccines against Toxoplasma gondii: new developments and perspectives. Expert Rev Vaccines. 2013; 12:1287–1299. PMID: 24093877.34. Ghaffarifar F. Strategies of DNA vaccines against toxoplasmosis. Rev Med Microbiol. 2015; 26:88–90.
Article35. Hoseinian Khosroshahi K, Ghaffarifar F, D'Souza S, Sharifi Z, Dalimi A. Evaluation of the immune response induced by DNA vaccine cocktail expressing complete SAG1 and ROP2 genes against toxoplasmosis. Vaccine. 2011; 29:778–783. PMID: 21095254.
Article36. Naserifar R, Ghaffarifar F, Dalimi A, Sharifi Z, Solhjoo K, Hosseinian Khosroshahi K. Evaluation of immunogenicity of cocktail DNA vaccine containing plasmids encoding complete GRA5, SAG1, and ROP2 antigens of Toxoplasma gondii in BALB/C mice. Iran J Parasitol. 2015; 10:590–598. PMID: 26811726.37. Jongert E, Roberts CW, Gargano N, Forster-Waldl E, Petersen E. Vaccines against Toxoplasma gondii: challenges and opportunities. Mem Inst Oswaldo Cruz. 2009; 104:252–266.
Article38. Buxton D. Toxoplasmosis: the first commercial vaccine. Parasitol Today. 1993; 9:335–337. PMID: 15463799.
Article39. Dlugonska H. Toxoplasma rhoptries: unique secretory organelles and source of promising vaccine proteins for immunoprevention of toxoplasmosis. J Biomed Biotechnol. 2008; 2008:632424. PMID: 18670609.40. Wang S, Hassan IA, Liu X, et al. Immunological changes induced by Toxoplasma gondii glutathione-S-transferase (TgGST) delivered as a DNA vaccine. Res Vet Sci. 2015; 99:157–164. PMID: 25648285.
Article41. Hassan IA, Wang S, Xu L, Yan R, Song X, Li X. Immunoglobulin and cytokine changes induced following immunization with a DNA vaccine encoding Toxoplasma gondii selenium-dependent glutathione reductase protein. Exp Parasitol. 2014; 146:1–10. PMID: 25173485.
Article42. Chu D, Moroda M, Piao LX, Aosai F. CTL induction by DNA vaccine with Toxoplasma gondii-HSP70 gene. Parasitol Int. 2014; 63:408–416. PMID: 24448159.
Article43. Zhao G, Zhou A, Lu G, et al. Identification and characterization of Toxoplasma gondii aspartic protease 1 as a novel vaccine candidate against toxoplasmosis. Parasit Vectors. 2013; 6:175. PMID: 23768047.
Article44. Gong P, Huang X, Yu Q, et al. The protective effect of a DNA vaccine encoding the Toxoplasma gondii cyclophilin gene in BALB/c mice. Parasite Immunol. 2013; 35:140–146. PMID: 23278173.45. Chen J, Huang SY, Zhou DH, et al. DNA immunization with eukaryotic initiation factor-2alpha of Toxoplasma gondii induces protective immunity against acute and chronic toxoplasmosis in mice. Vaccine. 2013; 31:6225–6231. PMID: 24183979.46. Chen J, Huang SY, Li ZY, et al. Protective immunity induced by a DNA vaccine expressing eIF4A of Toxoplasma gondii against acute toxoplasmosis in mice. Vaccine. 2013; 31:1734–1739. PMID: 23370151.
Article47. Uboldi AD, McCoy JM, Blume M, et al. Regulation of starch stores by a Ca(2+)-dependent protein kinase is essential for viable cyst development in Toxoplasma gondii. Cell Host Microbe. 2015; 18:670–681. PMID: 26651943.
Article48. Peixoto L, Chen F, Harb OS, et al. Integrative genomic approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host Microbe. 2010; 8:208–218. PMID: 20709297.
Article49. Billker O, Lourido S, Sibley LD. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 2009; 5:612–622. PMID: 19527888.
Article50. Nagamune K, Sibley LD. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the apicomplexa. Mol Biol Evol. 2006; 23:1613–1627. PMID: 16751258.
Article51. Moreno SN, Docampo R. Calcium regulation in protozoan parasites. Curr Opin Microbiol. 2003; 6:359–364. PMID: 12941405.
Article52. Zhang NZ, Huang SY, Zhou DH, et al. Protective immunity against Toxoplasma gondii induced by DNA immunization with the gene encoding a novel vaccine candidate: calcium-dependent protein kinase 3. BMC Infect Dis. 2013; 13:512. PMID: 24176018.
Article53. Zhang NZ, Xu Y, Wang M, et al. Vaccination with Toxoplasma gondii calcium-dependent protein kinase 6 and rhoptry protein 18 encapsulated in poly(lactide-co-glycolide) microspheres induces long-term protective immunity in mice. BMC Infect Dis. 2016; 16:168. PMID: 27090890.
Article54. Chen J, Li ZY, Petersen E, Liu WG, Zhu XQ. Co-administration of interleukins 7 and 15 with DNA vaccine improves protective immunity against Toxoplasma gondii. Exp Parasitol. 2016; 162:18–23. PMID: 26706605.
Article55. Zhang NZ, Huang SY, Xu Y, et al. Evaluation of immune responses in mice after DNA immunization with putative Toxoplasma gondii calcium-dependent protein kinase 5. Clin Vaccine Immunol. 2014; 21:924–929. PMID: 24789795.
Article56. Chen J, Li ZY, Huang SY, et al. Protective efficacy of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) adjuvated with recombinant IL-15 and IL-21 against experimental toxoplasmosis in mice. BMC Infect Dis. 2014; 14:487. PMID: 25192845.
Article57. Morlon-Guyot J, Berry L, Chen CT, Gubbels MJ, Lebrun M, Daher W. The Toxoplasma gondii calcium-dependent protein kinase 7 is involved in early steps of parasite division and is crucial for parasite survival. Cell Microbiol. 2014; 16:95–114. PMID: 24011186.58. Chen K, Wang JL, Huang SY, Yang WB, Zhu WN, Zhu XQ. Immune responses and protection after DNA vaccination against Toxoplasma gondii calcium-dependent protein kinase 2 (TgCDPK2). Parasite. 2017; 24:41. PMID: 29119944.59. Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004; 117:503–514. PMID: 15137943.
Article60. Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010; 465:359–362. PMID: 20485436.
Article61. Wang JL, Huang SY, Li TT, Chen K, Ning HR, Zhu XQ. Evaluation of the basic functions of six calcium-dependent protein kinases in Toxoplasma gondii using CRISPR-Cas9 system. Parasitol Res. 2016; 115:697–702. PMID: 26499803.
Article62. Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines. 2016; 15:313–329. PMID: 26707950.
Article63. Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines. 2012; 11:189–209. PMID: 22309668.
Article64. Doria-Rose NA, Haigwood NL. DNA vaccine strategies: candidates for immune modulation and immunization regimens. Methods. 2003; 31:207–216. PMID: 14511953.
Article65. Ojo KK, Larson ET, Keyloun KR, et al. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat Struct Mol Biol. 2010; 17:602–607. PMID: 20436472.
Article66. Zhang NZ, Huang SY, Zhou DH, Xu Y, He JJ, Zhu XQ. Identification and bioinformatic analysis of a putative calcium-dependent protein kinase (CDPK6) from Toxoplasma gondii. Genet Mol Res. 2014; 13:10669–10677. PMID: 25526188.
Article67. McCoy JM, Whitehead L, van Dooren GG, Tonkin CJ. TgCDPK3 regulates calcium-dependent egress of Toxoplasma gondii from host cells. PLoS Pathog. 2012; 8:e1003066. PMID: 23226109.
Article68. Garrison E, Treeck M, Ehret E, et al. A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog. 2012; 8:e1003049. PMID: 23209419.
Article69. Sayles PC, Gibson GW, Johnson LL. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect Immun. 2000; 68:1026–1033. PMID: 10678903.70. Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998; 11:569–588. PMID: 9767056.71. Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988; 240:516–518. PMID: 3128869.72. Dubensky TW Jr, Liu MA, Ulmer JB. Delivery systems for gene-based vaccines. Mol Med. 2000; 6:723–732. PMID: 11071268.
Article73. Bureau MF, Naimi S, Torero Ibad R, et al. Intramuscular plasmid DNA electrotransfer: biodistribution and degradation. Biochim Biophys Acta. 2004; 1676:138–148. PMID: 14746908.74. Greenland JR, Letvin NL. Chemical adjuvants for plasmid DNA vaccines. Vaccine. 2007; 25:3731–3741. PMID: 17350735.
Article75. Montomoli E, Piccirella S, Khadang B, Mennitto E, Camerini R, De Rosa A. Current adjuvants and new perspectives in vaccine formulation. Expert Rev Vaccines. 2011; 10:1053–1061. PMID: 21806399.
Article76. Khosroshahi KH, Ghaffarifar F, Sharifi Z, et al. Comparing the effect of IL-12 genetic adjuvant and alum non-genetic adjuvant on the efficiency of the cocktail DNA vaccine containing plasmids encoding SAG-1 and ROP-2 of Toxoplasma gondii. Parasitol Res. 2012; 111:403–411. PMID: 22350714.
Article77. Xue M, He S, Zhang J, Cui Y, Yao Y, Wang H. Comparison of cholera toxin A2/B and murine interleukin-12 as adjuvants of Toxoplasma multi-antigenic SAG1-ROP2 DNA vaccine. Exp Parasitol. 2008; 119:352–357. PMID: 18442818.
Article78. Liu Q, Wang F, Wang G, et al. Toxoplasma gondii: immune response and protective efficacy induced by ROP16/GRA7 multicomponent DNA vaccine with a genetic adjuvant B7-2. Hum Vaccin Immunother. 2014; 10:184–191. PMID: 24096573.79. Wang PY, Yuan ZG, Petersen E, et al. Protective efficacy of a Toxoplasma gondii rhoptry protein 13 plasmid DNA vaccine in mice. Clin Vaccine Immunol. 2012; 19:1916–1920. PMID: 23015648.
Article80. Dooms H, Abbas AK. Control of CD4+ T-cell memory by cytokines and costimulators. Immunol Rev. 2006; 211:23–38. PMID: 16824114.81. Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005; 115:1177–1187. PMID: 15841203.
Article82. Li ZY, Chen J, Petersen E, et al. Synergy of mIL-21 and mIL-15 in enhancing DNA vaccine efficacy against acute and chronic Toxoplasma gondii infection in mice. Vaccine. 2014; 32:3058–3065. PMID: 24690150.
Article83. Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008; 6:79–88. PMID: 18059289.
Article84. Bradley PJ, Ward C, Cheng SJ, et al. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J Biol Chem. 2005; 280:34245–34258. PMID: 16002398.
Article85. Liu Q, Li FC, Zhou CX, Zhu XQ. Research advances in interactions related to Toxoplasma gondii microneme proteins. Exp Parasitol. 2017; 176:89–98. PMID: 28286325.
Article86. Brossier F, David Sibley L. Toxoplasma gondii: microneme protein MIC2. Int J Biochem Cell Biol. 2005; 37:2266–2272. PMID: 16084754.
Article87. Shirota H, Klinman DM. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev Vaccines. 2014; 13:299–312. PMID: 24308579.
Article88. Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. J Control Release. 2003; 90:261–280. PMID: 12880694.
Article89. Jain S, O'Hagan DT, Singh M. The long-term potential of biodegradable poly(lactide-co-glycolide) microparticles as the next-generation vaccine adjuvant. Expert Rev Vaccines. 2011; 10:1731–1742. PMID: 22085176.
Article90. Xu Y, Zhang NZ, Wang M, et al. A long-lasting protective immunity against chronic toxoplasmosis in mice induced by recombinant rhoptry proteins encapsulated in poly (lactide-co-glycolide) microparticles. Parasitol Res. 2015; 114:4195–4203. PMID: 26243574.
Article91. Nabi H, Rashid I, Ahmad N, et al. Induction of specific humoral immune response in mice immunized with ROP18 nanospheres from Toxoplasma gondii. Parasitol Res. 2017; 116:359–370. PMID: 27785602.
Article92. Chuang SC, Ko JC, Chen CP, Du JT, Yang CD. Encapsulation of chimeric protein rSAG1/2 into poly(lactide-co-glycolide) microparticles induces long-term protective immunity against Toxoplasma gondii in mice. Exp Parasitol. 2013; 134:430–437. PMID: 23624036.
Article93. Flower DR, Macdonald IK, Ramakrishnan K, Davies MN, Doytchinova IA. Computer aided selection of candidate vaccine antigens. Immunome Res. 2010; 6(Suppl 2):S1.
Article94. Romano P, Giugno R, Pulvirenti A. Tools and collaborative environments for bioinformatics research. Brief Bioinform. 2011; 12:549–561. PMID: 21984743.
Article95. Wang Y, Wang G, Cai J, Yin H. Review on the identification and role of Toxoplasma gondii antigenic epitopes. Parasitol Res. 2016; 115:459–468. PMID: 26581372.
Article96. Zhou J, Wang L, Lu G, et al. Epitope analysis and protection by a ROP19 DNA vaccine against Toxoplasma gondii. Parasite. 2016; 23:17. PMID: 27055564.97. Cao A, Liu Y, Wang J, et al. Toxoplasma gondii: vaccination with a DNA vaccine encoding T- and B-cell epitopes of SAG1, GRA2, GRA7 and ROP16 elicits protection against acute toxoplasmosis in mice. Vaccine. 2015; 33:6757–6762. PMID: 26518401.
Article98. Cong H, Gu QM, Yin HE, et al. Multi-epitope DNA vaccine linked to the A2/B subunit of cholera toxin protect mice against Toxoplasma gondii. Vaccine. 2008; 26:3913–3921. PMID: 18555564.
Article99. Cong H, Yuan Q, Zhao Q, et al. Comparative efficacy of a multi-epitope DNA vaccine via intranasal, peroral, and intramuscular delivery against lethal Toxoplasma gondii infection in mice. Parasit Vectors. 2014; 7:145. PMID: 24685150.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In-depth computational analysis of calcium-dependent protein kinase 3 of Toxoplasma gondii provides promising targets for vaccination
- Immunoinformatic analysis of immunogenic B- and T-cell epitopes of MIC4 protein to designing a vaccine candidate against Toxoplasma gondii through an in-silico approach
- Gefitinib Inhibits the Growth of Toxoplasma gondii in HeLa Cells
- Protease activity of 80 kDa protein secreted from the apicomplexan parasite Toxoplasma gondii
- Antigenic properties of dense granule antigen 12 protein using bioinformatics tools in order to improve vaccine design against Toxoplasma gondii