J Periodontal Implant Sci.
2017 Dec;47(6):402-415. 10.5051/jpis.2017.47.6.402.
Evaluation of the periodontal regenerative properties of patterned human periodontal ligament stem cell sheets
- Affiliations
-
- 1Department of Periodontology, Chonbuk National University School of Dentistry and Institute of Oral Bioscience, Jeonju, Korea. grayheron@hanmail.net
- 2Department of Bioengineering, University of Washington, Seattle, WA, USA.
- 3Institute for Stem Cell and Regenerative Medicine, University of Washington, Seattle, WA, USA.
- 4Center for Cardiovascular Biology, University of Washington, Seattle, WA, USA.
- 5Research Institute of Clinical Medicine, Chonbuk National University, Jeonju, Korea.
- 6Biomedical Research Institute, Chonbuk National University Hospital, Jeonju, Korea.
- KMID: 2399746
- DOI: http://doi.org/10.5051/jpis.2017.47.6.402
Abstract
- PURPOSE
The aim of this study was to determine the effects of patterned human periodontal ligament stem cell (hPDLSC) sheets fabricated using a thermoresponsive substratum.
METHODS
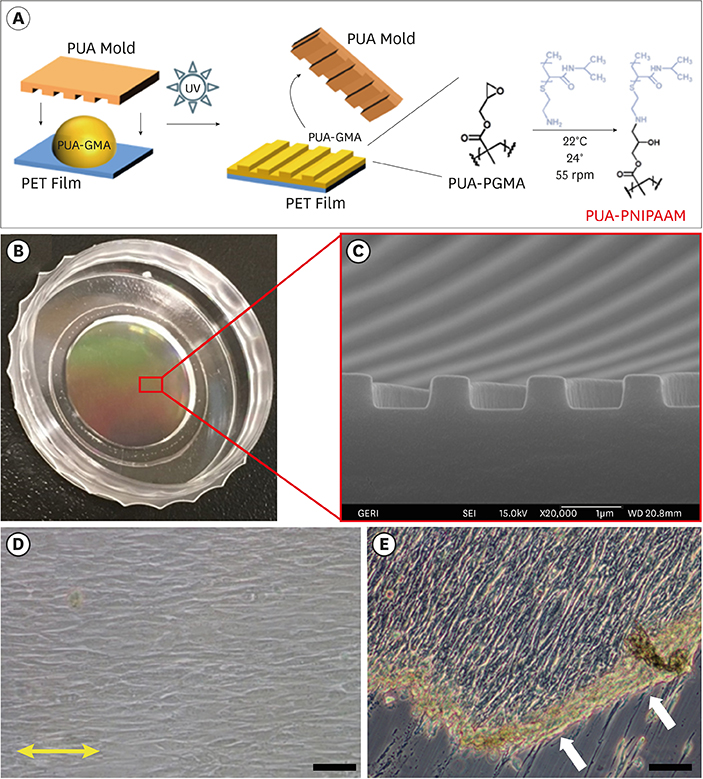
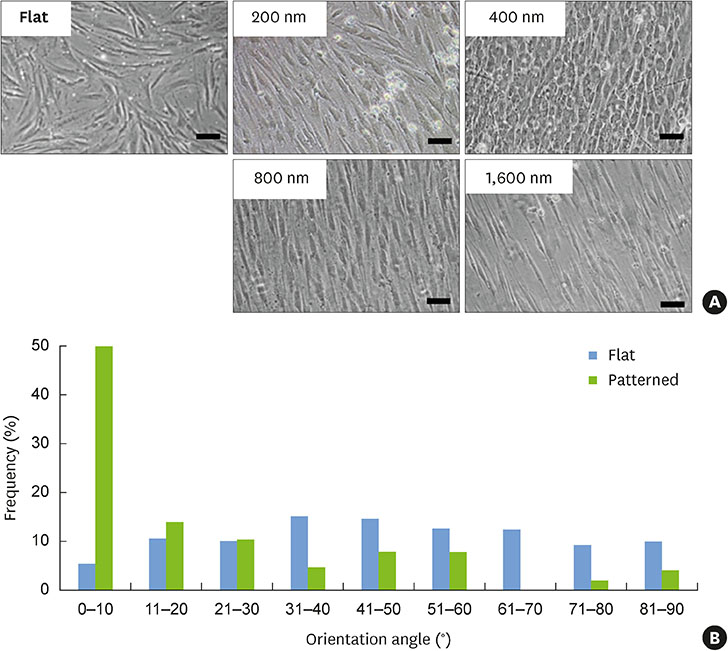
In this study, we fabricated patterned hPDLSC sheets using nanotopographical cues to modulate the alignment of the cell sheet.
RESULTS
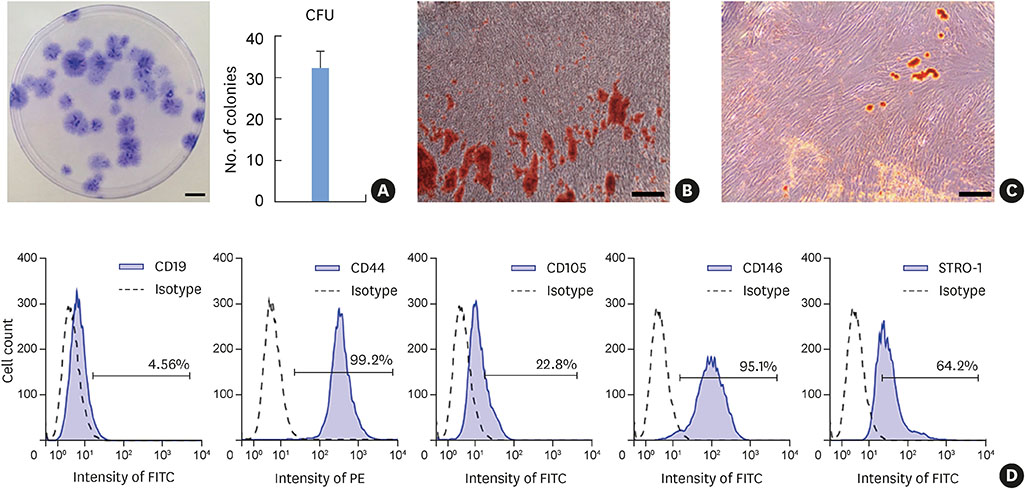
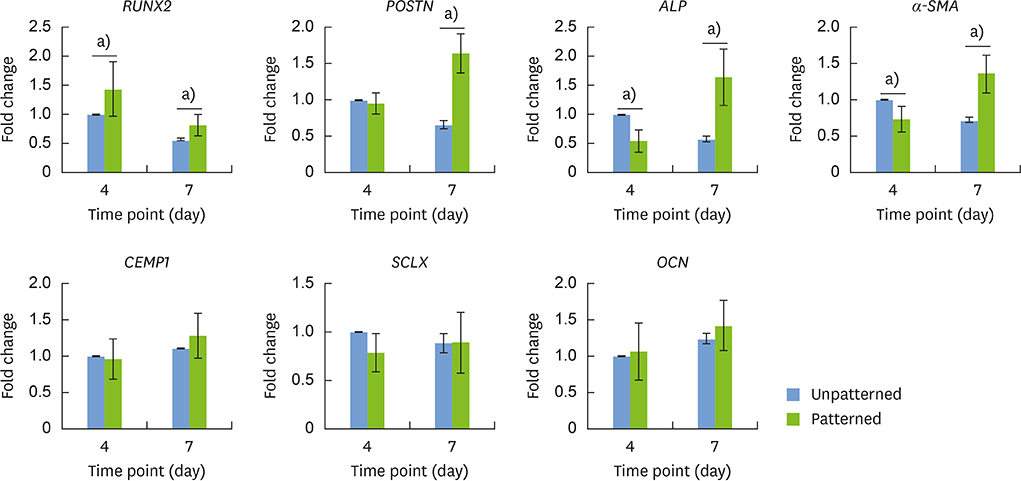
The hPDLSCs showed rapid monolayer formation on various surface pattern widths. Compared to cell sheets grown on flat surfaces, there were no significant differences in cell attachment and growth on the nanopatterned substratum. However, the patterned hPDLSC sheets showed higher periodontal ligamentogenesis-related gene expression in early stages than the unpatterned cell sheets.
CONCLUSIONS
This experiment confirmed that patterned cell sheets provide flexibility in designing hPDLSC sheets, and that these stem cell sheets may be candidates for application in periodontal regenerative therapy.
MeSH Terms
Figure
Reference
-
1. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999; 4:1–6.
Article2. Apicella A, Heunemann P, Dejace L, Marascio M, Plummer CJ, Fischer P. Scaffold requirements for periodontal regeneration with enamel matrix derivative proteins. Colloids Surf B Biointerfaces. 2017; 156:221–226.
Article3. Reynolds MA, Kao RT, Camargo PM, Caton JG, Clem DS, Fiorellini JP, et al. Periodontal regeneration - intrabony defects: a consensus report from the AAP Regeneration Workshop. J Periodontol. 2015; 86:S105–S107.
Article4. Greenwell H. Committee on Research, Science and Therapy. American Academy of Periodontology. Position paper: guidelines for periodontal therapy. J Periodontol. 2001; 72:1624–1628.5. Hasegawa M, Yamato M, Kikuchi A, Okano T, Ishikawa I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005; 11:469–478.
Article6. Bright R, Hynes K, Gronthos S, Bartold PM. Periodontal ligament-derived cells for periodontal regeneration in animal models: a systematic review. J Periodontal Res. 2015; 50:160–172.
Article7. Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007; 10:149–160.
Article8. Ishikawa I, Iwata T, Washio K, Okano T, Nagasawa T, Iwasaki K, et al. Cell sheet engineering and other novel cell-based approaches to periodontal regeneration. Periodontol 2000. 2009; 51:220–238.
Article9. Xu Q, Li B, Yuan L, Dong Z, Zhang H, Wang H, et al. Combination of platelet-rich plasma within periodontal ligament stem cell sheets enhances cell differentiation and matrix production. J Tissue Eng Regen Med. 2017; 11:627–636.
Article10. Carter SD, Costa PF, Vaquette C, Ivanovski S, Hutmacher DW, Malda J. Additive biomanufacturing: an advanced approach for periodontal tissue regeneration. Ann Biomed Eng. 2017; 45:12–22.
Article11. Okano T, Bae YH, Jacobs H, Kim SW. Thermally on-off switching polymers for drug permeation and release. J Control Release. 1990; 11:255–265.
Article12. Li M, Feng C, Gu X, He Q, Wei F. Effect of cryopreservation on proliferation and differentiation of periodontal ligament stem cell sheets. Stem Cell Res Ther. 2017; 8:77.
Article13. Guo S, Kang J, Ji B, Guo W, Ding Y, Wu Y, et al. Periodontal-derived mesenchymal cell sheets promote periodontal regeneration in inflammatory microenvironment. Tissue Eng Part A. 2017; 23:585–596.
Article14. Wang Z, Feng Z, Wu G, Bai S, Dong Y, Zhao Y. In vitro studies on human periodontal ligament stem cell sheets enhanced by enamel matrix derivative. Colloids Surf B Biointerfaces. 2016; 141:102–111.
Article15. Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007; 6:997–1003.
Article16. Kim DH, Provenzano PP, Smith CL, Levchenko A. Matrix nanotopography as a regulator of cell function. J Cell Biol. 2012; 197:351–360.
Article17. McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011; 10:637–644.
Article18. Gao H, Li B, Zhao L, Jin Y. Influence of nanotopography on periodontal ligament stem cell functions and cell sheet based periodontal regeneration. Int J Nanomedicine. 2015; 10:4009–4027.19. Jiao A, Trosper NE, Yang HS, Kim J, Tsui JH, Frankel SD, et al. Thermoresponsive nanofabricated substratum for the engineering of three-dimensional tissues with layer-by-layer architectural control. ACS Nano. 2014; 8:4430–4439.
Article20. Kim K, Yi T, Yun JH. Maintained stemness of human periodontal ligament stem cells isolated after prolonged storage of extracted teeth. J Periodontol. 2016; 87:e148–e158.
Article21. Farag A, Vaquette C, Theodoropoulos C, Hamlet SM, Hutmacher DW, Ivanovski S. Decellularized periodontal ligament cell sheets with recellularization potential. J Dent Res. 2014; 93:1313–1319.
Article22. Hu J, Cao Y, Xie Y, Wang H, Fan Z, Wang J, et al. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res Ther. 2016; 7:130.
Article23. Dan H, Vaquette C, Fisher AG, Hamlet SM, Xiao Y, Hutmacher DW, et al. The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials. 2014; 35:113–122.
Article24. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155.
Article25. Lemaitre M, Monsarrat P, Blasco-Baque V, Loubières P, Burcelin R, Casteilla L, et al. Periodontal tissue regeneration using syngeneic adipose-derived stromal cells in a mouse model. Stem Cells Transl Med. 2017; 6:656–665.
Article26. Wang ZS, Feng ZH, Wu GF, Bai SZ, Dong Y, Chen FM, et al. The use of platelet-rich fibrin combined with periodontal ligament and jaw bone mesenchymal stem cell sheets for periodontal tissue engineering. Sci Rep. 2016; 6:28126.
Article27. Feng R, Lengner C. Application of stem cell technology in dental regenerative medicine. Adv Wound Care (New Rochelle). 2013; 2:296–305.
Article28. Du J, Shan Z, Ma P, Wang S, Fan Z. Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration. J Dent Res. 2014; 93:183–188.
Article29. Owaki T, Shimizu T, Yamato M, Okano T. Cell sheet engineering for regenerative medicine: current challenges and strategies. Biotechnol J. 2014; 9:904–914.
Article30. Kim JH, Kang MS, Eltohamy M, Kim TH, Kim HW. Dynamic mechanical and nanofibrous topological combinatory cues designed for periodontal ligament engineering. PLoS One. 2016; 11:e0149967.
Article31. Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, et al. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010; 31:2163–2175.
Article32. Jiang W, Li L, Zhang D, Huang S, Jing Z, Wu Y, et al. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015; 25:240–252.
Article33. Gauvin R, Parenteau-Bareil R, Larouche D, Marcoux H, Bisson F, Bonnet A, et al. Dynamic mechanical stimulations induce anisotropy and improve the tensile properties of engineered tissues produced without exogenous scaffolding. Acta Biomater. 2011; 7:3294–3301.
Article34. Yu N, Prodanov L, te Riet J, Yang F, Walboomers XF, Jansen JA. Regulation of periodontal ligament cell behavior by cyclic mechanical loading and substrate nanotexture. J Periodontol. 2013; 84:1504–1513.
Article35. San Miguel SM, Fatahi MR, Li H, Igwe JC, Aguila HL, Kalajzic I. Defining a visual marker of osteoprogenitor cells within the periodontium. J Periodontal Res. 2010; 45:60–70.
Article36. Kalajzic Z, Li H, Wang LP, Jiang X, Lamothe K, Adams DJ, et al. Use of an alpha-smooth muscle actin GFP reporter to identify an osteoprogenitor population. Bone. 2008; 43:501–510.
Article37. Jin H, Choung HW, Lim KT, Jin B, Jin C, Chung JH, et al. Recombinant human plasminogen activator inhibitor-1 promotes cementogenic differentiation of human periodontal ligament stem cells. Tissue Eng Part A. 2015; 21:2817–2828.
Article38. Zhu B, Liu W, Liu Y, Zhao X, Zhang H, Luo Z, et al. Jawbone microenvironment promotes periodontium regeneration by regulating the function of periodontal ligament stem cells. Sci Rep. 2017; 7:40088.
Article39. Kadkhoda Z, Safarpour A, Azmoodeh F, Adibi S, Khoshzaban A, Bahrami N. Histopathological comparison between bone marrow- and periodontium-derived stem cells for bone regeneration in rabbit calvaria. Int J Organ Transplant Med. 2016; 7:9–18.40. Scott A, Danielson P, Abraham T, Fong G, Sampaio AV, Underhill TM. Mechanical force modulates scleraxis expression in bioartificial tendons. J Musculoskelet Neuronal Interact. 2011; 11:124–132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autologous Stem Cell Application in Periodontal Regeneration Technique (SAI-PRT) Using PDLSCs Directly From an Extracted Tooth...An Insight
- Comparison of Gene Expression from Supernumerary Dental Pulp and Periodontal Ligament Stem Cells
- Stem cell properties of cells derived from canine periodontal ligament
- Immunomodulatory effect of canine periodontal ligament stem cells on allogenic and xenogenic peripheral blood mononuclear cells
- Effects of nitric oxide on the proliferation and differentiation of human periodontal ligament cells