J Periodontal Implant Sci.
2010 Dec;40(6):265-270. 10.5051/jpis.2010.40.6.265.
Immunomodulatory effect of canine periodontal ligament stem cells on allogenic and xenogenic peripheral blood mononuclear cells
- Affiliations
-
- 1Department of Periodontology and Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. ymlee@snu.ac.kr
- 2Department of Periodontics, Asan Medical Center, Ulsan University College of Medicine, Seoul, Korea.
- KMID: 1783570
- DOI: http://doi.org/10.5051/jpis.2010.40.6.265
Abstract
- PURPOSE
The aim of this study was to investigate the immunomodulatory effects of canine periodontal ligament stem cells on allogenic and xenogenic immune cells in vitro.
METHODS
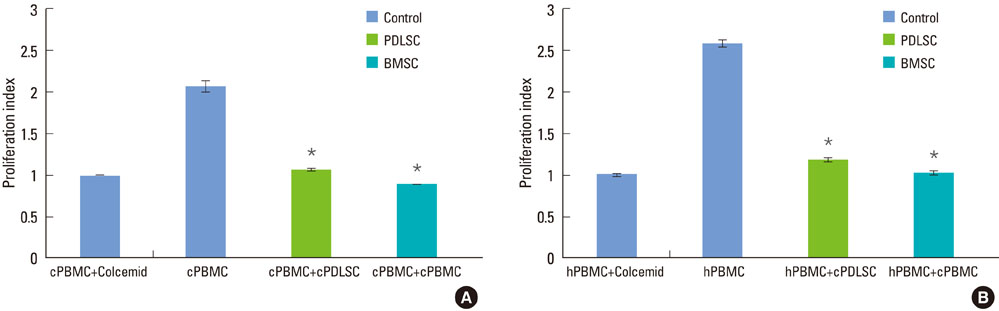
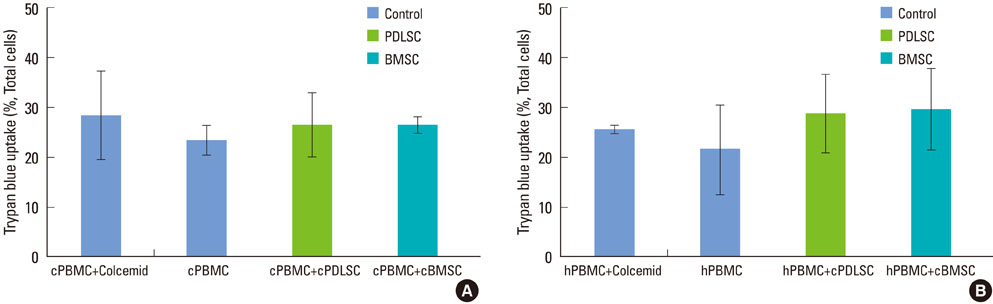
Mixed cell cultures consisting of canine stem cells (periodontal ligament stem cells and bone marrow stem cells) and allogenic canine/xenogenic human peripheral blood mononuclear cells (PBMCs) were established following the addition of phytohemagglutinin. The proliferation of PBMCs was evaluated using the MTS assay. The cell division of PBMCs was analyzed using the CFSE assay. The apoptosis of PBMCs was assessed using the trypan blue uptake method.
RESULTS
Periodontal ligament stem cells and bone marrow stem cells inhibited the proliferation of allogenic and xenogenic PBMCs. Both periodontal ligament stem cells and bone marrow stem cells suppressed the cell division of PBMCs despite the existence of a mitogen. No significant differences in the percentages of apoptotic PBMCs were found among the groups.
CONCLUSIONS
Canine periodontal ligament stem cells have an immunomodulatory effect on allogenic and xenogenic PBMCs. This effect is not a product of apoptosis of PBMCs but is caused by the inhibition of cell division of PBMCs.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Anti-inflammatory effect of (-)-epigallocatechin-3-gallate on Porphyromonas gingivalis lipopolysaccharide-stimulated fibroblasts and stem cells derived from human periodontal ligament
Im-Hee Jung, Dong-Eun Lee, Jeong-Ho Yun, Ah-Ran Cho, Chang-Sung Kim, Yoon-Jeong You, Sung-Jo Kim, Seong-Ho Choi
J Periodontal Implant Sci. 2012;42(6):185-195. doi: 10.5051/jpis.2012.42.6.185.
Reference
-
1. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002; 99:3838–3843.
Article2. Liu J, Lu XF, Wan L, Li YP, Li SF, Zeng LY, et al. Suppression of human peripheral blood lymphocyte proliferation by immortalized mesenchymal stem cells derived from bone marrow of Banna Minipig inbred-line. Transplant Proc. 2004; 36:3272–3275.
Article3. Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009; 1176:101–117.
Article4. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155.
Article5. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006; 1:e79.
Article6. Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008; 34:166–171.
Article7. Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001; 288:413–419.
Article8. Liu D, Yu J, Chen H, Reichman R, Wu H, Jin X. Statistical determination of threshold for cellular division in the CFSE-labeling assay. J Immunol Methods. 2006; 312:126–136.
Article9. Lin NH, Gronthos S, Mark Bartold P. Stem cells and future periodontal regeneration. Periodontol 2000. 2009; 51:239–251.
Article10. Jhin MJ, Kim YS, Kim SH, Kim KH, Lee CW, Koo KT, et al. Investigation of postnatal stem cells from canine dental tissue and bone marrow. J Korean Acad Periodontol. 2009; 39:119–128.
Article11. Kim SH, Kim KH, Seo BM, Koo KT, Kim TI, Seol YJ, et al. Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: a pilot study. J Periodontol. 2009; 80:1815–1823.
Article12. Choumerianou DM, Dimitriou H, Kalmanti M. Stem cells: promises versus limitations. Tissue Eng Part B Rev. 2008; 14:53–60.
Article13. Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009; 219:667–676.
Article14. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007; 110:3499–3506.
Article15. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003; 31:890–896.
Article16. Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003; 102:3837–3844.
Article17. Puissant N, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Brit J Haematol. 2005; 129:118–129.
Article18. Bruder SP, Horowitz MC, Mosca JD, Haynesworth SE. Monoclonal antibodies reactive with human osteogenic cell surface antigens. Bone. 1997; 21:225–235.
Article19. Nagatomo K, Komaki M, Sekiya I, Sakaguchi Y, Noguchi K, Oda S, et al. Stem cell properties of human periodontal ligament cells. J Periodontal Res. 2006; 41:303–310.
Article20. Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003; 76:1208–1213.
Article21. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003; 75:389–397.
Article22. Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006; 24:74–85.
Article23. Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005; 105:2214–2219.
Article24. Poznansky MC, Olszak IT, Foxall R, Evans RH, Luster AD, Scadden DT. Active movement of T cells away from a chemokine. Nat Med. 2000; 6:543–548.
Article25. Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002; 20:795–823.
Article26. Roper RL, Ludlow JW, Phipps RP. Prostaglandin E2 inhibits B lymphocyte activation by a cAMP-dependent mechanism: PGE-inducible regulatory proteins. Cell Immunol. 1994; 154:296–308.
Article27. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002; 196:459–468.
Article28. Rasmusson I. Immune modulation by mesenchymal stem cells. Experimental Cell Research. 2006; 312:2169–2179.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem cell properties of cells derived from canine periodontal ligament
- Comparison of Gene Expression from Supernumerary Dental Pulp and Periodontal Ligament Stem Cells
- Investigation of postnatal stem cells from canine dental tissue and bone marrow
- Autologous Stem Cell Application in Periodontal Regeneration Technique (SAI-PRT) Using PDLSCs Directly From an Extracted Tooth...An Insight
- An Immune-compromised Method for Tooth Transplantation Using Adult Bone Marrow Stromal Cells and Embryonic Tooth Germ