Asia Pac Allergy.
2013 Jul;3(3):186-193. 10.5415/apallergy.2013.3.3.186.
T cell transcriptional factors in allergic rhinitis and its association with clinical features
- Affiliations
-
- 1Department of Otorhinolaryngology, Dankook University College of Medicine, Cheonan 330-715, Korea. jihunmo@gmail.com
- 2Medical Laser and Device Research Center, Dankook University College of Medicine, Cheonan 330-715, Korea.
- KMID: 2397312
- DOI: http://doi.org/10.5415/apallergy.2013.3.3.186
Abstract
- BACKGROUND
Th2 cells are crucially important in allergic disease and the possible involvement of Treg and Th17 cells has not been clearly identified.
OBJECTIVE
To identify the mRNA expression of T cell transcription factors in nasal mucosa in patients with allergic rhinitis (AR) and to reveal their correlations with clinical features.
METHODS
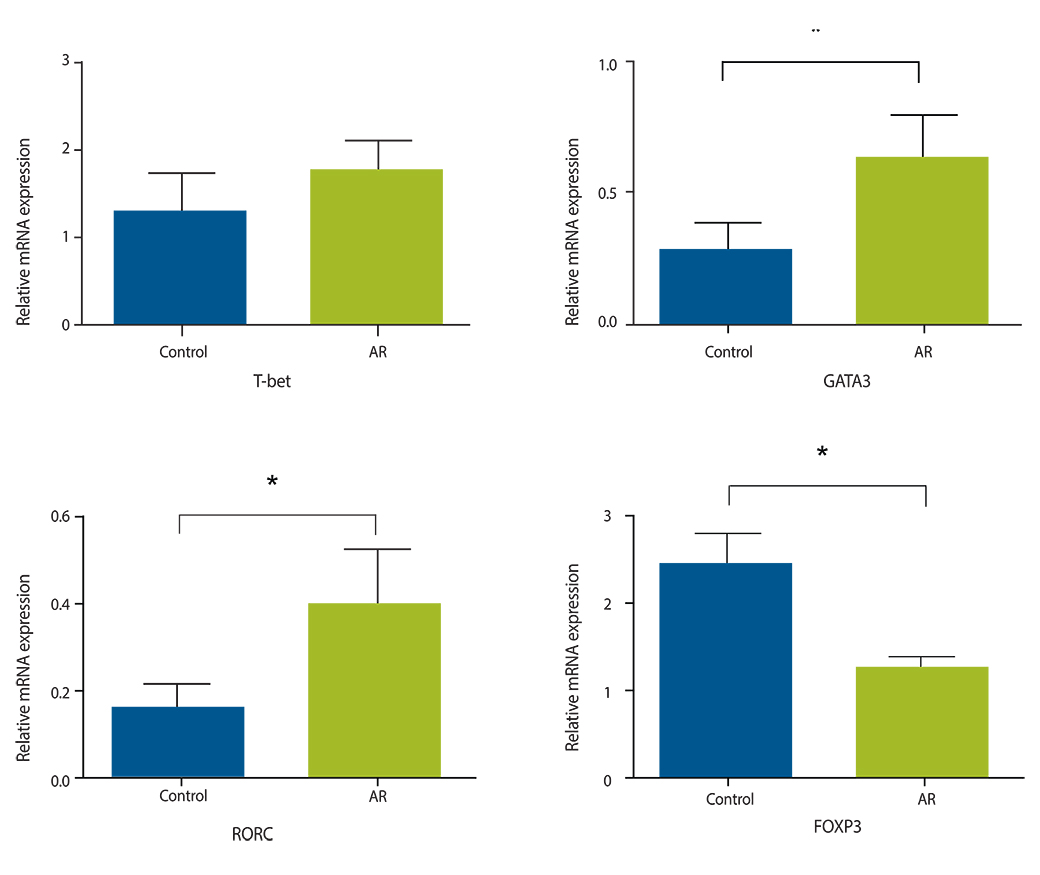
Eighteen patients with AR and 12 controls with turbinate hypertrophy were included. mRNA expression of the following transcriptional factors in nasal mucosa were measured by quantitative polymerase chain reaction; T-bet (Th1), GATA3 (Th2), retinoic acid-related orphan receptor C (RORC; Th17), and forkhead box P3 (Foxp3; Treg). mRNA expression was compared among groups and correlation between mRNA expression level and clinical features (rhinitis symptoms, eosinophil count, and IgE) were also investigated.
RESULTS
GATA3 and RORC were significantly increased and Foxp3 was significantly decreased in the AR group. Moderate-to-severe AR group also had increased expression of GATA3 and RORC than mild AR group, suggesting severity of AR influence expression of transcription factors. Correlation analysis showed that none of these transcription factors were associated with severity of clinical symptoms, eosinophil counts and skin prick test severity and that IgE level was significantly correlated with expression level of GATA3 and RORC, suggesting an association of IgE production with Th2 and Th17 cells.
CONCLUSION
Increased mRNA expression of GATA3 (Th2), increased expression of RORC and decreased expression of Foxp3 may be important in pathogenesis of AR. GATA3 and RORC may be closed related with IgE level.
Keyword
MeSH Terms
Figure
Reference
-
1. Oboki K, Ohno T, Saito H, Nakae S. Th17 and allergy. Allergol Int. 2008; 57:121–134.
Article2. Nouri-Aria KT, Durham SR. Regulatory T cells and allergic disease. Inflamm Allergy Drug Targets. 2008; 7:237–252.
Article3. Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000; 165:6107–6115.
Article4. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006; 126:1121–1133.5. Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, Lam CW. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001; 125:177–183.6. Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001; 108:430–438.
Article7. Barczyk A, Pierzchala W, Sozañska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003; 97:726–733.
Article8. Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010; 151:297–307.
Article9. Ciprandi G, De Amici M, Murdaca G, Fenoglio D, Ricciardolo F, Marseglia G, Tosca M. Serum interleukin-17 levels are related to clinical severity in allergic rhinitis. Allergy. 2009; 64:1375–1378.
Article10. Ciprandi G, Fenoglio D, De Amici M, Quaglini S, Negrini S, Filaci G. Serum IL-17 levels in patients with allergic rhinitis. J Allergy Clin Immunol. 2008; 122:650–651.e2.
Article11. Liu F, Zhang J, Liu Y, Zhang N, Holtappels G, Lin P, Liu S, Bachert C. Inflammatory profiles in nasal mucosa of patients with persistent vs intermittent allergic rhinitis. Allergy. 2010; 65:1149–1157.12. Xu G, Mou Z, Jiang H, Cheng L, Shi J, Xu R, Oh Y, Li H. A possible role of CD4+CD25+ T cells as well as transcription factor Foxp3 in the dysregulation of allergic rhinitis. Laryngoscope. 2007; 117:876–880.
Article13. Lee SM, Gao B, Dahl M, Calhoun K, Fang D. Decreased FoxP3 gene expression in the nasal secretions from patients with allergic rhinitis. Otolaryngol Head Neck Surg. 2009; 140:197–201.
Article14. Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004; 199:1567–1575.
Article15. Shi HZ, Li S, Xie ZF, Qin XJ, Qin X, Zhong XN. Regulatory CD4+CD25+ T lymphocytes in peripheral blood from patients with atopic asthma. Clin Immunol. 2004; 113:172–178.
Article16. Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004; 363:608–615.
Article17. Brüstle A, Heink S, Huber M, Rosenplänter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007; 8:958–966.
Article18. Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, Bernstein JA, Huston DP, Liu YJ. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010; 207:2479–2491.
Article19. Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008; 452:773–776.
Article20. Al Khatib S, Keles S, Garcia-Lloret M, Karakoc-Aydiner E, Reisli I, Artac H, Camcioglu Y, Cokugras H, Somer A, Kutukculer N, Yilmaz M, Ikinciogullari A, Yegin O, Yüksek M, Genel F, Kucukosmanoglu E, Baki A, Bahceciler NN, Rambhatla A, Nickerson DW, McGhee S, Barlan IB, Chatila T. Defects along the T(H)17 differentiation pathway underlie genetically distinct forms of the hyper IgE syndrome. J Allergy Clin Immunol. 2009; 124:342–348. 348.e1–348.e5.
Article